NCERT Science Class 9 Chapter 2 Question Answer Solutions – Is Matter Around Us Pure FREE PDF Download
Page 15
Q. 1. What is meant by a Substance?
Answer:-
🧪 What is a Substance?
A substance is a type of matter that has a definite composition and distinct properties. It is pure, meaning it is made up of only one kind of particle and has uniform properties throughout.
🔍 Characteristics of a Substance:
✅ Fixed Composition – A substance has the same chemical composition throughout.
✅ Cannot be Broken by Physical Means – It cannot be separated into different components by physical methods like filtration or distillation.
✅ Distinct Properties – Every substance has unique physical and chemical properties, such as melting point, boiling point, and density.
🌿 Types of Substances:
1️⃣ Elements – Pure substances made of only one type of atom (e.g., Oxygen 🫁, Gold 🏆, Hydrogen 💧).
2️⃣ Compounds – Pure substances made of two or more different elements chemically combined (e.g., Water 💦 (H₂O), Carbon dioxide 🍃 (CO₂)).
📌 Key Point to Remember:
A substance always has a fixed composition and specific properties, distinguishing it from mixtures.
Q. 2. List the points of differences between homogeneous and heterogenous mixtures.
Answer:-
🌍 Differences Between Homogeneous and Heterogeneous Mixtures
Homogeneous and heterogeneous mixtures are two types of mixtures that differ in their composition and appearance. Below are the key differences:
| 🔍 Feature | 🧪 Homogeneous Mixture | 🌿 Heterogeneous Mixture |
|---|---|---|
| ✅ Definition | A mixture in which the components are uniformly distributed throughout. | A mixture in which the components are not evenly distributed and can be easily distinguished. |
| 👀 Appearance | Looks same throughout as it has a uniform composition. | Different substances are clearly visible, making it non-uniform. |
| 🏺 Examples | Salt solution 🧂💧, Air 🌬️, Sugar in water 🍯 | Sand and iron filings 🏖️, Oil and water 🛢️💦, Salad 🥗 |
| 🔬 Separation | Cannot be separated easily by physical methods. | Can be separated easily using physical methods like filtration or handpicking. |
| ⚖️ Composition | Same proportion of components throughout. | Different proportions of components in different parts of the mixture. |
📌 Key Point to Remember:
Homogeneous mixtures have uniform composition, while heterogeneous mixtures have visible differences in composition. 💯✨
Page – 18
Q. 1. Differentiate between homogenous and heterogenous mixtures with examples.
Answer:-
🌍 Differences Between Homogeneous and Heterogeneous Mixtures
Homogeneous and heterogeneous mixtures differ in their composition and appearance. Below is a structured comparison:
| 🔍 Feature | 🧪 Homogeneous Mixture | 🌿 Heterogeneous Mixture |
|---|---|---|
| ✅ Definition | A mixture in which components are uniformly distributed throughout. | A mixture in which components are not evenly distributed and can be easily distinguished. |
| 👀 Appearance | Looks same throughout as it has a uniform composition. | Different substances are clearly visible, making it non-uniform. |
| 🏺 Examples | Salt solution 🧂💧, Air 🌬️, Sugar in water 🍯 | Sand and iron filings 🏖️, Oil and water 🛢️💦, Salad 🥗 |
| 🔬 Separation | Cannot be separated easily by physical methods. | Can be separated easily using physical methods like filtration or handpicking. |
| ⚖️ Composition | Same proportion of components throughout. | Different proportions of components in different parts of the mixture. |
📌 Key Point to Remember:
Homogeneous mixtures have uniform composition, while heterogeneous mixtures have visible differences in composition. 💯✨
Q. 2 How are sol, solution and suspension different from each other?
Answer:-
🌊 Differences Between Sol, Solution, and Suspension
Sol, solution, and suspension are different types of mixtures based on the size of particles and their stability. Below is a structured comparison:
| 🔍 Feature | 🧪 Sol | 💧 Solution | 🌿 Suspension |
|---|---|---|---|
| ✅ Definition | A colloidal solution where solid particles are dispersed in a liquid. | A homogeneous mixture where solute dissolves completely in the solvent. | A heterogeneous mixture where particles remain dispersed but settle over time. |
| 🔬 Particle Size | 1-1000 nm (colloidal range). | Less than 1 nm (very small). | Greater than 1000 nm (large particles). |
| 👀 Appearance | Cloudy but stable, does not settle. | Transparent and uniform, looks the same throughout. | Cloudy and unstable, particles settle over time. |
| 📌 Example | Ink 🖊️, Paint 🎨, Blood 🩸 | Salt solution 🧂💧, Sugar in water 🍯 | Sand in water 🏖️💦, Chalk powder in water 🏺 |
| ⚖️ Separation | Cannot be separated by filtration. | Cannot be separated by filtration. | Can be separated easily by filtration. |
| 🔥 Tyndall Effect | Shows the Tyndall effect (light scattering). | Does not show the Tyndall effect. | Shows the Tyndall effect strongly. |
📌 Key Point to Remember:
- Sol is a colloidal mixture with medium-sized particles.
- Solution is a homogeneous mixture with tiny particles that dissolve completely.
- Suspension is a heterogeneous mixture with large particles that settle over time.
Q. 3. To make a saturated solution, 36g of sodium chloride is dissolved in 100g of water at 293K. Find its concentration at this temperature.
Answer:-
🧪 Calculating the Concentration of a Saturated Solution
To find the concentration of the sodium chloride (NaCl) solution, we use the formula:
[Concentration] = Mass of solute\Mass of solution x100
📌 Given Data:
✅ Mass of solute (NaCl) = 36g
✅ Mass of solvent (Water) = 100g
✅ Mass of solution = Mass of solute + Mass of solvent = 36g + 100g = 136g
🧮 Calculation:
[Concentration] = 36/136 x100
[ = 26.47% ]
🎯 Final Answer:
🔹 The concentration of the saturated NaCl solution at 293K is 26.47% 💯✨
Page – 19
Q. 1. Classify the following as chemical or physical changes:
- Cutting of trees
- melting of butter in a pan
- rusting of almirah
- boiling of water to form steam
- Passing of electric current, through water and the water breaking down into hydrogen and oxygen gases.
- dissolving common salt in water
- Making a fruit salad with raw fruits, and burning of paper and wood.
Answer:-
🔥 Classification of Changes: Chemical vs. Physical
Understanding the difference between physical and chemical changes is essential. A physical change only affects the form of a substance, while a chemical change results in the formation of a new substance with different properties.
📌 Classification of the Given Changes:
✅ Physical Changes – No new substance is formed:
1️⃣ Cutting of trees 🌲✂️
2️⃣ Melting of butter in a pan 🧈🔥
3️⃣ Boiling of water to form steam 💨💦
4️⃣ Dissolving common salt in water 🧂💧
5️⃣ Making a fruit salad with raw fruits 🍎🍌🥗
☑️ Chemical Changes – A new substance is formed with different properties:
1️⃣ Rusting of almirah 🏠⚙️ (Formation of iron oxide)
2️⃣ Passing electric current through water, breaking it into hydrogen and oxygen 🔥💧 (Electrolysis of water)
3️⃣ Burning of paper and wood 📄🔥🌳 (Formation of ash and gases)
🌟 Key Takeaway:
- Physical changes are reversible and do not alter the composition of the substance.
- Chemical changes are irreversible and lead to the formation of a new substance.
Q. 2. Try segregating the things around you as pure substances or mixtures.
Answer:-
🏠 Classification of Everyday Items: Pure Substances vs. Mixtures
To classify objects around us, we need to understand:
✅ Pure Substance – Contains only one type of particle and has a fixed composition.
✅ Mixture – Consists of two or more substances mixed physically, and composition can vary.
🧪 Pure Substances (Elements & Compounds)
1️⃣ Iron nails 🔩 – Made of a single element (Iron, Fe)
2️⃣ Distilled water 💧 – Pure H₂O without impurities
3️⃣ Salt (Sodium Chloride) 🧂 – A compound with fixed composition (NaCl)
4️⃣ Oxygen gas 🫁 – Contains only O₂ molecules
🌿 Mixtures (Homogeneous & Heterogeneous)
1️⃣ Tea ☕ – A homogeneous mixture of water, sugar, and tea extract
2️⃣ Air 🌬️ – A homogeneous mixture of gases (O₂, N₂, CO₂, etc.)
3️⃣ Sand and gravel 🏖️ – A heterogeneous mixture of different particles
4️⃣ Fruit salad 🍎🥭🍌 – A heterogeneous mixture of different fruits
📌 Key Takeaway:
- Pure substances have fixed compositions and properties.
- Mixtures contain two or more substances that can be separated by physical means.
Back Excercises Questions
Q. 1. Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water
(b) Ammonium chloride from a mixture containing sodium
chloride and ammonium chloride
(c) Small pieces of metal in the engine oil of a car
(d) Different pigments from an extract of flower petals
(e) Butter from curd
(f) Oil from water
(g) Tea leaves from tea
(h) Iron pins from sand
(i) Wheat grains from husk
(j) Fine mud particles suspended in water
Answer:-
🔬 Separation Techniques for Different Mixtures
Below are the appropriate separation techniques for each case:
| 🔍 Mixture | ⚗️ Separation Technique | ✨ Description |
|---|---|---|
| (a) Sodium chloride from its solution in water 🧂💧 | Evaporation 🌡️ | The water is evaporated, leaving behind salt crystals. |
| (b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride 🏺 | Sublimation ❄️ | Ammonium chloride sublimes upon heating, leaving sodium chloride behind. |
| (c) Small pieces of metal in the engine oil of a car 🚗 | Filtration using a magnet 🧲 | Metal pieces are attracted to a magnet, separating them from oil. |
| (d) Different pigments from an extract of flower petals 🌺 | Chromatography 🖊️ | Pigments are separated based on their different solubilities in a solvent. |
| (e) Butter from curd 🧈 | Centrifugation 🌀 | Spinning curd at high speed separates butter. |
| (f) Oil from water 🛢️💦 | Decantation & Separating Funnel ⚖️ | Oil floats above water and is removed using a separating funnel. |
| (g) Tea leaves from tea 🍵 | Filtration 🏺 | Tea leaves are separated by filtering through a sieve or strainer. |
| (h) Iron pins from sand 🏖️ | Magnetic Separation 🧲 | Iron pins are attracted to a magnet and separated from sand. |
| (i) Wheat grains from husk 🌾 | Winnowing 🍃 | Light husk is separated from heavier wheat grains by blowing air. |
| (j) Fine mud particles suspended in water 💦 | Sedimentation & Decantation 🚰 | Mud settles down due to gravity, and clean water is decanted. |
📌 Key Takeaway:
Each separation technique is based on differences in physical properties like solubility, density, magnetism, and volatility.
Q. 2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:-
☕ Steps for Making Tea
Making tea involves the preparation of a solution, where a solute (tea leaves, sugar, etc.) dissolves in a solvent (water). Here are the steps:
1️⃣ Boiling the Solvent – Take water (solvent) in a pan and heat it until it starts boiling. 🔥💧
2️⃣ Adding Solute – Add tea leaves and sugar (solute) to the boiling water. Sugar dissolves completely, while tea leaves remain insoluble. 🫖🍯
3️⃣ Formation of Solution – The tea leaves release color and flavor, forming a solution of tea. 🌿☕
4️⃣ Filtration – Pour the tea through a sieve or strainer. This separates the filtrate (liquid tea) from the residue (leftover tea leaves). 🏺🫗
5️⃣ Enjoying the Tea – The filtered tea can be served hot and enjoyed! 😊✨
📌 Key Points:
✅ Sugar is soluble in water, dissolving completely.
✅ Tea leaves are insoluble, and remain as residue after filtration.
✅ The final tea solution is the filtrate that is consumed.
Q. 3. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
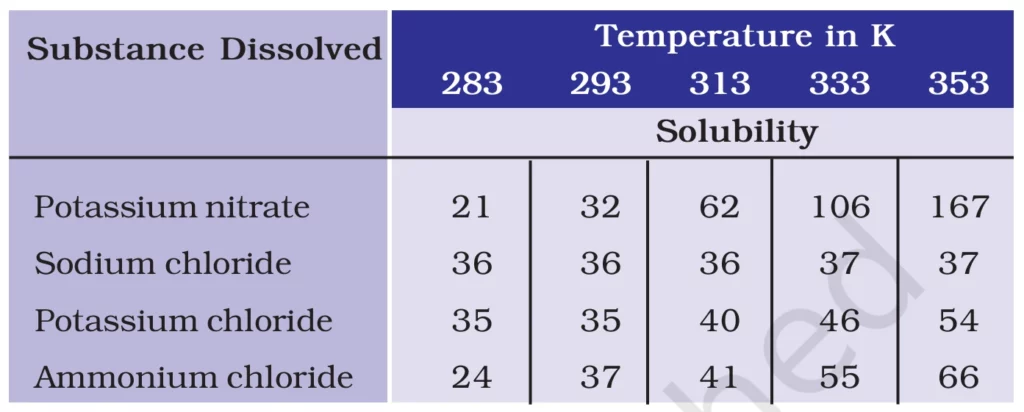
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Solution:-
🌡️ Solubility Analysis and Calculations
(a) Mass of Potassium Nitrate Needed for a Saturated Solution in 50g of Water at 313K
- From the table, solubility of potassium nitrate at 313K = 62g per 100g water.
- Using the formula:
[ \text{Mass of solute} = \frac{\text{Solubility at given temperature}}{100} \times \text{Mass of water} ]
[ = \frac{62}{100} \times 50 ]
[ = 31g ]
✅ Answer: 31g of potassium nitrate is needed to make a saturated solution in 50g of water at 313K.
(b) Observation When a Saturated Solution of Potassium Chloride at 353K is Cooled
- Solubility of potassium chloride decreases as temperature drops.
- Since the solution was saturated at 353K, some potassium chloride will crystallize and settle as it cools. ❄️💧
✅ Observation: Potassium chloride crystals will form as the solution cools due to reduced solubility.
(c) Solubility of Each Salt at 293K & Most Soluble Salt
From the table at 293K:
- Potassium nitrate → 32g per 100g water
- Sodium chloride → 36g per 100g water
- Potassium chloride → 35g per 100g water
- Ammonium chloride → 37g per 100g water
✅ Most soluble salt at 293K: Ammonium chloride 🏆 (37g per 100g water).
(d) Effect of Temperature on Solubility of Salts
- Generally, solubility of solids in liquids increases with temperature 🔥📈.
- Exceptions exist where some salts show minimal change or even slight decrease.
- Example: Potassium nitrate solubility drastically increases with temperature, while sodium chloride shows very little change.
✅ Key takeaway: Higher temperature increases solubility of most salts but the degree of change varies.
Q. 4. Explain the following giving examples.
(a) Saturated solution
(b) Pure substance
(c) Colloid
(d) Suspension
Answer:-
🧪 Explanation of Key Scientific Terms
Here are the definitions and examples of the given concepts:
(a) Saturated Solution 🌊
A saturated solution is a solution in which no more solute can be dissolved at a given temperature. The solution has reached its maximum capacity to dissolve the solute.
✅ Example:
- If 36g of sodium chloride (NaCl) is dissolved in 100g of water at 293K, the solution becomes saturated. Any extra salt will remain undissolved at the bottom. 🧂💧
(b) Pure Substance 🔬
A pure substance is a material that has a fixed composition and uniform properties throughout. It is made up of only one kind of particle and cannot be separated into simpler substances by physical methods.
✅ Examples:
- Elements – Gold 🏆 (Au), Oxygen 🫁 (O₂)
- Compounds – Water 💦 (H₂O), Carbon dioxide 🍃 (CO₂)
(c) Colloid 🌫️
A colloid is a heterogeneous mixture in which particles remain dispersed throughout the medium but do not settle down. The particle size is between 1nm and 1000nm, and colloids exhibit the Tyndall Effect (scattering of light).
✅ Examples:
- Milk 🥛 (Fat particles dispersed in water)
- Fog 🌫️ (Water droplets dispersed in air)
- Blood 🩸 (Cell particles suspended in plasma)
(d) Suspension 🏺
A suspension is a heterogeneous mixture in which large particles are dispersed in a liquid but settle down over time. The particle size is greater than 1000nm, and suspensions also show the Tyndall Effect.
✅ Examples:
- Chalk powder in water 🏺💦
- Sand in water 🏖️🌊
- Flour in water 🍞💧
📌 Key Takeaway:
- Saturated Solution → No more solute dissolves 🧂💧
- Pure Substance → Fixed composition, cannot be separated 🏆
- Colloid → Particles dispersed, do not settle, shows Tyndall Effect 🌫️
- Suspension → Particles settle down, large size 🏺
Q. 5. Classify each of the following as a homogeneous or heterogeneous mixture.
soda water, wood, air, soil, vinegar, filtered tea.
Answer:-
🌍 Classification of Mixtures: Homogeneous vs. Heterogeneous
Mixtures can be classified based on how their components are distributed:
✅ Homogeneous Mixtures – Composition is uniform throughout, and the components are not visibly distinguishable.
❌ Heterogeneous Mixtures – Composition is non-uniform, and different substances are clearly visible.
📌 Classification Table
| 🔍 Substance | 🧪 Type of Mixture | ✨ Reason |
|---|---|---|
| Soda water 🥤💨 | Homogeneous ✅ | Dissolved carbon dioxide is uniformly mixed in water. |
| Wood 🌲 | Heterogeneous ❌ | Made of different compounds like cellulose, lignin, and water, which are not uniformly distributed. |
| Air 🌬️ | Homogeneous ✅ | A uniform mixture of gases like oxygen, nitrogen, and carbon dioxide. |
| Soil 🏞️ | Heterogeneous ❌ | Contains different components like sand, clay, minerals, and organic matter, which are visibly distinct. |
| Vinegar 🍾 | Homogeneous ✅ | Acetic acid is completely dissolved in water, forming a uniform solution. |
| Filtered tea 🍵 | Homogeneous ✅ | All components dissolve uniformly, making it look the same throughout. |
📌 Key Takeaway
- Homogeneous mixtures have a uniform composition throughout, with no visible separation of components.
- Heterogeneous mixtures have distinct components that can be seen separately.
Q. 6. How would you confirm that a colourless liquid given to you is pure water?
Answer:-
🔬 Confirming the Purity of Water
To verify whether a colorless liquid is pure water, we can perform various tests based on its physical and chemical properties.
✅ Step 1: Check Boiling and Freezing Points
- Pure water boils at 100°C 🌡️🔥 under normal atmospheric pressure.
- Pure water freezes at 0°C ❄️🌡️.
If the liquid does not boil or freeze at these exact temperatures, it may contain impurities.
✅ Step 2: Observe Electrical Conductivity ⚡💧
- Pure water is a poor conductor of electricity.
- If the liquid conducts electricity, it may contain dissolved salts or impurities.
✅ Step 3: Evaporation Test 🌊☀️
- Place a few drops on a clean surface and allow it to evaporate.
- If any residue is left behind, it is not pure water and contains impurities like salts or minerals.
✅ Step 4: pH Test 🏺🔬
- Pure water has a neutral pH of 7.
- Use pH paper or a pH meter to check.
- If the pH is less than 7 → Acidic impurities ⚡
- If the pH is greater than 7 → Basic impurities 🏺
📌 Key Takeaway:
Pure water should:
✅ Boil at 100°C and freeze at 0°C.
✅ Be a poor conductor of electricity.
✅ Leave no residue after evaporation.
✅ Have a neutral pH of 7.
Q. 7. Which of the following materials fall in the category of a “pure substance”?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air
Solution:-
🧪 Classification of Pure Substances and Mixtures
A pure substance contains only one kind of particle and has a fixed composition. It cannot be separated into simpler substances by physical means.
✅ Pure Substances
1️⃣ Ice ❄️ – Solid form of water (H₂O), a compound with fixed composition.
2️⃣ Iron 🔩 – An element (Fe) with uniform properties.
3️⃣ Hydrochloric Acid ⚗️ – A compound (HCl) with fixed composition.
4️⃣ Calcium Oxide 🏺 – A compound (CaO) with definite properties.
5️⃣ Mercury 🌡️ – An element (Hg), with uniform composition.
❌ Mixtures (Not Pure Substances)
1️⃣ Milk 🥛 – A colloidal mixture of water, fat, proteins, and sugars.
2️⃣ Brick 🧱 – Made of clay, sand, and other materials, making it a heterogeneous mixture.
3️⃣ Wood 🌳 – Composed of cellulose, lignin, and water, making it a heterogeneous mixture.
4️⃣ Air 🌬️ – A homogeneous mixture of gases like oxygen, nitrogen, and carbon dioxide.
📌 Key Takeaway:
✅ Pure substances include elements and compounds with fixed composition.
❌ Mixtures have variable composition and can be separated by physical means.
Q. 8. Identify the solutions among the following mixtures.
(a) Soil
(b) Sea water
(c) Air
(d) Coal
(e) Soda water
Solution:-
🌊 Identification of Solutions
A solution is a homogeneous mixture where the solute is completely dissolved in the solvent. Let’s classify the given mixtures:
✅ Solutions (Homogeneous Mixtures)
1️⃣ Sea water 🌊 – Salt and minerals are uniformly dissolved in water.
2️⃣ Air 🌬️ – A homogeneous mixture of gases like oxygen, nitrogen, and carbon dioxide.
3️⃣ Soda water 🥤 – Carbon dioxide gas is dissolved uniformly in water under pressure.
❌ Not Solutions (Heterogeneous Mixtures)
1️⃣ Soil 🏞️ – A heterogeneous mixture of sand, minerals, organic matter, and clay.
2️⃣ Coal 🔥 – Composed of different substances, making it heterogeneous.
📌 Key Takeaway:
✅ Solutions are uniform mixtures where substances dissolve completely.
❌ Heterogeneous mixtures have visible differences in composition.
Q. 9. Which of the following will show “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution
Solution:-
🔬 Tyndall Effect – Which Mixtures Show It?
The Tyndall Effect is the scattering of light by particles in a mixture. Colloidal solutions show this effect because their particle size is large enough to scatter light, whereas true solutions do not.
✅ Mixtures That Show Tyndall Effect
1️⃣ Milk 🥛 – A colloid, with fat particles suspended in water.
2️⃣ Starch solution 🍚💧 – A colloidal solution, with large starch particles dispersed in water.
❌ Mixtures That Do NOT Show Tyndall Effect
1️⃣ Salt solution 🧂💧 – A true solution, with completely dissolved salt molecules.
2️⃣ Copper sulphate solution 🔵💧 – A true solution, where copper sulfate dissolves completely without scattering light.
📌 Key Takeaway:
✅ Colloids like milk and starch solution show the Tyndall Effect.
❌ True solutions like salt solution and copper sulphate solution do not scatter light.
Q. 10. Classify the following into elements, compounds and mixtures.
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
(h) Coal
(i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood
Answer:-
🌍 Classification of Substances
Here is a structured classification of the given substances into elements, compounds, and mixtures:
| 🔍 Substance | 🧪 Classification | ✨ Reason |
|---|---|---|
| (a) Sodium 🧂 | Element ✅ | A pure element (Na) with uniform properties. |
| (b) Soil 🏞️ | Mixture ❌ | Contains sand, minerals, organic matter, and water. |
| (c) Sugar solution 🍯💧 | Mixture ❌ | Sugar dissolves in water to form a homogeneous mixture. |
| (d) Silver 🏆 | Element ✅ | A pure element (Ag) with uniform properties. |
| (e) Calcium carbonate 🏺 | Compound ✅ | Composed of calcium (Ca), carbon (C), and oxygen (O₃). |
| (f) Tin 🔩 | Element ✅ | A pure element (Sn) with a fixed composition. |
| (g) Silicon 🏗️ | Element ✅ | A pure element (Si) found in nature and electronics. |
| (h) Coal 🔥 | Mixture ❌ | Contains different carbon-based compounds and impurities. |
| (i) Air 🌬️ | Mixture ❌ | A homogeneous mixture of gases like oxygen, nitrogen, and carbon dioxide. |
| (j) Soap 🧼 | Mixture ❌ | Contains various compounds, fats, and alkali substances. |
| (k) Methane 🔥 | Compound ✅ | A pure compound (CH₄) with a fixed composition. |
| (l) Carbon dioxide 🍃 | Compound ✅ | A pure compound (CO₂) with uniform properties. |
| (m) Blood 🩸 | Mixture (Colloid) ❌ | Contains plasma, red blood cells, white blood cells, and platelets. |
📌 Key Takeaway:
✅ Elements – Pure substances made of one type of atom (e.g., Sodium, Silver, Silicon).
✅ Compounds – Pure substances made of two or more chemically combined elements (e.g., Calcium carbonate, Methane, Carbon dioxide).
❌ Mixtures – Composed of two or more substances mixed physically (e.g., Soil, Air, Blood).
Q. 11. Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle
Answer:-
🔬 Classification of Physical and Chemical Changes
A chemical change leads to the formation of new substances with different properties, while a physical change only affects the state or appearance of a substance without altering its composition.
✅ Chemical Changes – Formation of a New Substance
1️⃣ Growth of a plant 🌱 – Involves photosynthesis and biochemical reactions that create new compounds.
2️⃣ Rusting of iron ⚙️ – Iron reacts with oxygen and moisture to form iron oxide (Fe₂O₃·xH₂O).
3️⃣ Cooking of food 🍲 – Heat triggers chemical reactions, producing new substances with different flavors and textures.
4️⃣ Digestion of food 🍎 – Enzymes break down food into new chemical compounds for absorption.
5️⃣ Burning of a candle 🕯️🔥 – Wax undergoes combustion, forming carbon dioxide and water vapor.
☑️ Physical Changes – No New Substance Formed
1️⃣ Mixing of iron filings and sand 🏖️ – No reaction occurs; they can be physically separated by a magnet.
2️⃣ Freezing of water ❄️💧 – Only the state changes from liquid to solid, but the composition remains the same.
📌 Key Takeaway:
✅ Chemical changes result in the formation of new substances and are irreversible.
❌ Physical changes only alter the appearance or state and are usually reversible.