NCERT Science Class 9 Chapter 4 Question Answer Solutions – Structure oF The Atom FREE PDF Download
Page – 39
✨ Q.1. What are Canal Rays? ⚡🔬💡
Answer:-
✨ Canal Rays ⚡🔬
Definition:
Canal rays, also known as anode rays ➕🔋, are positively charged radiations that were discovered by Eugen Goldstein in 1886. These rays consist of positively charged particles, later identified as protons 🧪⚛️.
🔍 Discovery & Explanation
Goldstein observed that when a perforated cathode 🕳️ was used in a discharge tube filled with gas, a new type of rays moved in the opposite direction of cathode rays. Since these rays passed through the canals (holes) in the cathode, they were named Canal Rays 🚀✨.
⚡ Characteristics of Canal Rays
✅ Positively charged particles ➕🔋
✅ Move in the opposite direction of cathode rays 🔄
✅ Deflected by electric and magnetic fields 🧲⚡
✅ Have mass and occupy space ⚖️🌍
✅ Led to the discovery of protons 🏆🔬
🌟 Importance in Atomic Structure
The discovery of canal rays played a crucial role in understanding atomic structure 🧪⚛️. It helped scientists identify protons, which are present in the nucleus of an atom 🏡🔬.
💡 Conclusion: Canal rays are an essential discovery in atomic physics, as they provided evidence of positively charged particles in atoms, leading to the development of modern atomic models.
✨ Q.2. If an atom contains one electron ⚡➖ and one proton 🔋➕, will it carry any charge or not? ⚖️💡
Answer:-
⚛️ Charge on an Atom with One Electron and One Proton
An atom consists of subatomic particles 🧪🔬, including electrons ⚡➖ (negatively charged) and protons 🔋➕ (positively charged).
If an atom contains one electron ⚡ and one proton 🔋, the negative charge of the electron cancels out the positive charge of the proton. Since both charges are equal in magnitude but opposite in nature, the atom remains electrically neutral ⚖️🌍.
🔍 Key Explanation:
✅ Electron (-1 charge) + Proton (+1 charge) = Net charge = 0 ⚖️
✅ No overall charge on the atom 🚫⚡
✅ Example: A hydrogen atom 🏡⚛️ consists of one electron and one proton, making it neutral.
💡 Conclusion:
An atom with one electron and one proton does not carry any charge because the opposite charges balance each other. Hence, it is electrically neutral ⚖️✨.
Page – 41
✨ Q.1. On the basis of Thomson’s model ⚛️🔬 of an atom, explain how the atom is neutral ⚖️🌍 as a whole. 💡🔋
Answer:-
✨ Thomson’s Model of an Atom ⚛️🔬
According to J.J. Thomson, an atom is like a plum pudding 🍮 or a watermelon 🍉, where:
🔴 The positively charged matter (protons) is spread throughout the atom, just like the soft, red part of a watermelon.
⚫ The negatively charged electrons are embedded within it, just like the black seeds in a watermelon!
⚖️ Why is the Atom Neutral as a Whole? 🌍
An atom consists of:
🔋 Positively charged protons (+) spread uniformly.
⚡ Negatively charged electrons (−) scattered throughout.
Since the total positive charge is equal to the total negative charge, they balance each other out. 🌟 As a result, the atom remains electrically neutral ⚖️—neither positively nor negatively charged!
🎯 Board Exam Tip:
Write this answer neatly 🖊️, add a simple diagram 📊 of Thomson’s atomic model, and use proper scientific terms to get full marks ✅!
✨ Q.2. On the basis of Rutherford’s model 🔬⚛️ of an atom, which subatomic particle 🔋🧪 is present in the nucleus 🏡🌍 of an atom? 💡
Answer:-
✨ Rutherford’s Model of an Atom 🔬⚛️
The gold foil experiment 🌟 conducted by Ernest Rutherford led to the discovery of a new atomic model!
🏡 The Nucleus – A Dense Core 🌍
According to Rutherford:
🔹 An atom has a small, dense, positively charged nucleus 🏡 at its center.
🔹 Electrons ⚡ revolve around this nucleus in circular orbits, just like planets around the Sun! 🌞🪐
🔋 Which Subatomic Particle is Present in the Nucleus? 🧪
👉 The nucleus contains positively charged particles called protons ➕⚛️!
👉 Later, scientists discovered neutrons 🔬, which are also present inside the nucleus but carry no charge ⚪.
🌟 Board Exam Tip:
✅ Mention Rutherford’s gold foil experiment 🏆.
✅ Draw a simple labeled diagram 📊 to illustrate the atomic structure.
✅ Use precise scientific terms and maintain clarity in your explanation!
✨ Q.3. Draw a sketch 🖊️📜 of Bohr’s model ⚛️🔬 of an atom with three shells 🔄➰➰. 💡
Answer:-
✨ Q.4. What do you think 🤔💡 would be the observation 👀🔬 if the α-particle scattering experiment ⚛️✨ is carried out using a foil 🏗️📜 of a metal other than gold? 💭🔍
Answer:-
✨ Observations of the α-Particle Scattering Experiment Using a Different Metal Foil ⚛️✨
In Rutherford’s α-particle scattering experiment 🔬✨, gold foil 🏗️📜 was used because:
- Gold is highly malleable 🏆, allowing it to be beaten into extremely thin layers.
- Gold atoms are relatively large ⚛️, making it easier to observe scattering patterns.
💡 What Would Happen with Another Metal Foil? 🔍
If the experiment were carried out using a different metal foil 🏗️📜, the observations would depend on the metal’s atomic properties:
1️⃣ Similar Observations ✅
If the metal has a large atomic size ⚛️ and can be beaten into a thin foil, the results would be similar:
🔸 Most α-particles ⚡ would pass straight through 📡.
🔸 Some would deflect at small angles 🔄.
🔸 A very few would bounce back ⬅️ due to hitting the dense nucleus 🏡.
2️⃣ Different Observations ❌
If the metal has a smaller atomic size ⚛️ or cannot be made into a thin foil, then:
🔹 More α-particles might be deflected because of tightly packed nuclei 🔄.
🔹 Fewer α-particles would pass through 📡 if the metal is not as malleable.
🔹 Scattering patterns would differ, affecting Rutherford’s conclusions about the atomic nucleus! 🏡⚛️
🎯 Board Exam Tip:
✅ Mention why gold was used 🏗️📜.
✅ Explain how atomic properties affect the experiment 📊.
✅ Draw a simple labeled diagram ✏️⚛️ to show α-particle paths!
Page – 41
✨ Q.1. Name the three sub-atomic particles ⚛️🔬 of an atom. 🔋➖⚖️
Answer:-
✨ Three Sub-Atomic Particles of an Atom ⚛️🔬
An atom consists of three fundamental sub-atomic particles:
1️⃣ Proton ➕⚛️
🔹 Located in the nucleus 🏡🌍
🔹 Has a positive charge (+1) ⚡
🔹 Determines the atomic number of an element 🏷️🔢
2️⃣ Neutron ⚪🔬
🔹 Also found in the nucleus 🏡
🔹 Has no charge (neutral) ⚖️
🔹 Contributes to the mass of the atom ⚖️🧮
3️⃣ Electron ⚡➖
🔹 Revolves around the nucleus in shells or orbits 🔄➰
🔹 Has a negative charge (-1) ⚡
🔹 Responsible for chemical bonding and reactions 🧪💥
🎯 Board Exam Tip:
✅ Clearly define each particle with its charge, location, and role.
✅ Draw a labeled diagram ✏️📜 of an atom showing protons, neutrons, and electrons.
✅ Keep explanations precise and well-structured to secure full marks 💯🏆!
✨ Q.2. A helium atom ⚛️💨 has an atomic mass of 4 u ⚖️🔬 and two protons 🔋➕ in its nucleus 🏡🌍. How many neutrons ⚫💡 does it have?
Answer:-
✨ Number of Neutrons in a Helium Atom ⚛️💨
A helium atom has:
🔹 Atomic mass = 4 u ⚖️🔬
🔹 Number of protons = 2 🔋➕
🧮 Formula to Calculate Neutrons ⚫💡
Neutrons = Atomic mass – Number of protons
📌 Substituting values:
Neutrons = 4 u – 2 ➡ 2 neutrons ⚫⚫
✅ Final Answer:
A helium atom has 2 neutrons inside its nucleus 🏡🌍.
🎯 Board Exam Tip:
✅ Write the formula clearly.
✅ Show the step-by-step calculation 🧮.
✅ Keep the explanation neat & precise 🏆 for full marks 💯!
Page – 42
Q. 1. Write the distribution of electrons in carbon and sodium atoms.
Answer:-
✨ Electron Distribution in Carbon and Sodium Atoms ⚛️🔬
In an atom, electrons ⚡ are arranged in shells (energy levels) 🔄➰ around the nucleus 🏡.
🔹 Electron Distribution in Carbon (C) 🏗️⚛️
🔸 Atomic Number = 6 🔢
🔸 Number of Electrons ⚡ = 6
📌 Distribution:
K-shell (1st) ➡ 2 electrons ⚡⚡
L-shell (2nd) ➡ 4 electrons ⚡⚡⚡⚡
✅ Electronic configuration of Carbon: 2, 4
🔹 Electron Distribution in Sodium (Na) 🏗️⚛️
🔸 Atomic Number = 11 🔢
🔸 Number of Electrons ⚡ = 11
📌 Distribution:
K-shell (1st) ➡ 2 electrons ⚡⚡
L-shell (2nd) ➡ 8 electrons ⚡⚡⚡⚡⚡⚡⚡⚡
M-shell (3rd) ➡ 1 electron ⚡
✅ Electronic configuration of Sodium: 2, 8, 1
🎯 Board Exam Tip:
✅ Write atomic numbers and electron arrangements clearly 🖊️.
✅ Draw labeled diagrams ✏️📜 showing shells and electrons.
✅ Keep the explanation precise and structured 🏆 for full marks 💯!
Q. 2. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer:-
✨ Total Number of Electrons When K and L Shells Are Full ⚛️🔬
Electrons ⚡ in an atom are arranged in shells (energy levels) 🔄➰ based on the Bohr’s atomic model!
🏡 Maximum Electrons in K and L Shells 🔬
🔸 K-shell (1st energy level): Can hold 2 electrons ⚡⚡
🔸 L-shell (2nd energy level): Can hold 8 electrons ⚡⚡⚡⚡⚡⚡⚡⚡
✅ Total Number of Electrons
If both K and L shells are full, the atom will have:
📌 2 (K-shell) + 8 (L-shell) = 10 electrons ⚡✨
Thus, the atom contains 10 electrons in total when its K and L shells are completely filled! 🔟⚛️
🎯 Board Exam Tip:
✅ Clearly explain electron arrangement 🏡.
✅ Mention Bohr’s rule for maximum electrons in each shell! 🔬📖
✅ Draw a simple labeled diagram ✏️📜 showing K and L shell electron distribution.
Page – 44
Q. 1. How will you find the valency of chlorine, sulphur and magnesium?
Answer:-
✨ Finding the Valency of Chlorine, Sulphur, and Magnesium ⚛️🔬
Valency refers to the combining capacity of an atom 🌍🔗. It depends on the number of electrons in the outermost shell 🔄➰ of an atom.
🔹 Valency of Chlorine (Cl) 🏗️⚛️
🔸 Atomic Number = 17 🔢
🔸 Electronic Configuration = 2, 8, 7 ⚡⚡➰➰➰➰➰
📌 Valency = 8 – Outer electrons = 8 – 7 = 1
✅ Chlorine has a valency of 1 ➕✨, meaning it needs 1 electron to complete its octet!
🔹 Valency of Sulphur (S) 🏗️⚛️
🔸 Atomic Number = 16 🔢
🔸 Electronic Configuration = 2, 8, 6 ⚡⚡➰➰➰➰➰➰
📌 Valency = 8 – Outer electrons = 8 – 6 = 2
✅ Sulphur has a valency of 2 ➕✨, meaning it needs 2 electrons to complete its octet!
🔹 Valency of Magnesium (Mg) 🏗️⚛️
🔸 Atomic Number = 12 🔢
🔸 Electronic Configuration = 2, 8, 2 ⚡⚡➰➰
📌 Valency = Outer electrons = 2
✅ Magnesium has a valency of 2 ➕✨, meaning it can lose 2 electrons to become stable!
🎯 Board Exam Tip:
✅ Write electron configurations clearly 🖊️.
✅ Explain how valency is determined 🏆.
✅ Draw a simple labeled diagram ✏️📜 for visual clarity.
✅ Keep explanations structured and precise to secure full marks 💯🏆!
Page – 44
Q. 1. If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom? and (ii) what is the charge on the atom?
Answer:-
✨ Atomic Number & Charge of the Atom ⚛️🔬
Given:
🔹 Number of electrons = 8 ⚡
🔹 Number of protons = 8 🔋
(i) Atomic Number of the Atom 🔢⚛️
The atomic number of an element is the number of protons in its nucleus 🏡.
📌 Since the atom has 8 protons, its atomic number = 8 ✅.
Thus, the element is Oxygen (O) 🌬️!
(ii) Charge on the Atom ⚖️💡
- Protons (+) ➡ 8 positive charges ➕➕➕➕➕➕➕➕
- Electrons (-) ➡ 8 negative charges ➖➖➖➖➖➖➖➖
📌 Since positive and negative charges are equal, they cancel each other out, making the atom neutral ⚖️🌍!
🎯 Board Exam Tip:
✅ Write definitions clearly 🖊️.
✅ Show step-by-step explanation 🧮.
✅ Keep answers neat & structured for full marks 💯🏆!
Q. 2. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Answer:-
✨ Mass Number of Oxygen & Sulphur Atoms ⚛️🔬
The mass number of an atom is calculated as:
📌 Mass Number = Number of Protons + Number of Neutrons ⚖️💡
🔹 Mass Number of Oxygen (O) 🌬️⚛️
🔸 Atomic Number = 8 🔢
🔸 Protons = 8 ➕
🔸 Neutrons = 8 ⚪
📌 Mass Number = 8 + 8 = 16
✅ Oxygen has a mass number of 16 ⚖️🌍!
🔹 Mass Number of Sulphur (S) 🏗️⚛️
🔸 Atomic Number = 16 🔢
🔸 Protons = 16 ➕
🔸 Neutrons = 16 ⚪
📌 Mass Number = 16 + 16 = 32
✅ Sulphur has a mass number of 32 ⚖️🌍!
🎯 Board Exam Tip:
✅ Clearly define mass number 🏆.
✅ Show step-by-step calculations 🧮.
✅ Keep answers precise & well-structured to ensure full marks 💯🏆!
Page – 45
Q. 1. For the symbol H,D and T tabulate three sub-atomic particles found in each of them.
Answer:-
✨ Tabulation of Sub-Atomic Particles in Hydrogen (H), Deuterium (D), and Tritium (T) ⚛️🔬
Hydrogen, Deuterium (heavy hydrogen), and Tritium (radioactive hydrogen) are isotopes 🏗️ of the same element, differing only in their number of neutrons!
📜 Table of Sub-Atomic Particles
| Isotope ⚛️ | Protons 🔋➕ | Neutrons ⚫💡 | Electrons ⚡➖ |
|---|---|---|---|
| Hydrogen (H) 🌬️ | 1 | 0 | 1 |
| Deuterium (D) 🏗️ | 1 | 1 | 1 |
| Tritium (T) ⚡ | 1 | 2 | 1 |
🎯 Board Exam Tip:
✅ Mention that H, D, and T are isotopes of hydrogen! ⚛️
✅ Clearly define and differentiate them in terms of neutrons.
✅ Use a well-structured table 📊 for clarity.
✅ Keep answers neat, concise, and precise for full marks 💯🏆!
Q. 2. Write the electronic configuration of any one pair of isotopes and isobars.
Answer:-
✨ Electronic Configuration of Isotopes & Isobars ⚛️🔬
Isotopes are atoms of the same element 🏗️ but with different numbers of neutrons ⚫💡.
Isobars are atoms of different elements 🌍 but with the same mass number ⚖️!
🔹 Example of Isotopes: Carbon-12 (¹²C) & Carbon-14 (¹⁴C)
✅ Both belong to Carbon (C) 🏗️
✅ Atomic Number = 6 🔢 (Same for both isotopes)
✅ Electronic Configuration = 2, 4 ⚡➰➰
📌 Difference:
- ¹²C has 6 neutrons ⚪
- ¹⁴C has 8 neutrons ⚪⚪
Thus, isotopes have same electronic configuration but different neutron count! 🌟
🔹 Example of Isobars: Calcium-40 (⁴⁰Ca) & Argon-40 (⁴⁰Ar)
✅ Different elements (Ca & Ar) 🌍
✅ Same mass number = 40 ⚖️
✅ Different atomic numbers 🔢
📌 Electronic configurations:
- Calcium (⁴⁰Ca) ⚛️ ➡ 2, 8, 8, 2 ⚡➰➰➰➰
- Argon (⁴⁰Ar) 🌍 ➡ 2, 8, 8 ⚡➰➰➰
📌 Difference:
- Calcium has 20 protons & 20 neutrons 🔋⚪
- Argon has 18 protons & 22 neutrons 🔋⚪⚪
Thus, isobars have different electronic configurations but same mass number! 🌟
🎯 Board Exam Tip:
✅ Clearly define isotopes & isobars 🏆
✅ Show step-by-step electronic configurations 🧮
✅ Use structured tables or diagrams ✏️📜 for clarity
✅ Keep answers precise & well-organized to get full marks 💯🏆
Chapter Back Excercise Question Answer
Q. 1. Compare the properties of electrons, protons and neutrons.
Answer:-
✨ Comparison of Electrons, Protons, and Neutrons ⚛️🔬
Atoms are made up of three fundamental sub-atomic particles 🌍. Each particle has unique properties that determine the behavior of an atom!
📜 Tabular Comparison
| Property 🏗️ | Electron (⚡➖) | Proton (🔋➕) | Neutron (⚪) |
|---|---|---|---|
| Charge ⚖️ | Negative (-1) | Positive (+1) | Neutral (0) |
| Location 🏡 | Revolves around nucleus in shells 🔄➰ | Inside nucleus 🏡 | Inside nucleus 🏡 |
| Mass ⚖️ | Very small (≈1/1836 u) | 1 atomic mass unit (u) | 1 atomic mass unit (u) |
| Role 💡 | Responsible for chemical reactions ⚛️🧪 | Determines atomic number 🔢 | Adds to atomic mass & stability ⚖️🌍 |
🎯 Board Exam Tip:
✅ Use a neatly structured table 📊 for clarity!
✅ Mention charge, location, mass & role of each particle 🏆.
✅ Keep the explanation precise to secure full marks 💯!
✅ Draw a labeled atomic structure diagram ✏️📜 for a visual boost!
Q. 2. What are the limitations of J.J. Thomson’s model of the atom?
Answer:-
✨ Limitations of J.J. Thomson’s Model of the Atom ⚛️🔬
J.J. Thomson proposed the Plum Pudding Model 🍮 of an atom, where:
🔹 The atom was a positively charged sphere ⚡🌍.
🔹 Electrons ⚫ were embedded like raisins in a pudding 🍇.
However, this model had several limitations that led to new discoveries! 🚀
🔴 Major Limitations ❌
1️⃣ Could Not Explain Rutherford’s Experiment 🔬✨
📌 The α-particle scattering experiment showed that atoms have a dense nucleus 🏡, contradicting Thomson’s model!
2️⃣ Did Not Explain Atomic Structure ⚛️💡
📌 Thomson’s model could not explain how electrons move or why they don’t collapse into the nucleus.
3️⃣ Failed to Explain Nuclear Charge 🔋⚖️
📌 Rutherford’s model later proved that the positive charge is concentrated in the nucleus, not spread throughout the atom.
🎯 Board Exam Tip:
✅ Mention Thomson’s Plum Pudding Model 🍮⚛️.
✅ Clearly explain why Rutherford’s experiment disproved it 📜✨.
✅ Keep answers structured & precise for full marks 💯🏆!
Q. 3. What are the limitations of Rutherford’s model of the atom?
Answer:-
✨ Limitations of Rutherford’s Model of the Atom 🔬⚛️
Rutherford’s gold foil experiment 🌟 revealed the presence of a dense, positively charged nucleus 🏡. His atomic model explained how electrons revolve around the nucleus, but it had some major limitations! 🚀
🔴 Major Limitations of Rutherford’s Model ❌
1️⃣ Could Not Explain Stability of Electrons ⚡🔄
📌 According to classical physics, revolving electrons should lose energy and spiral into the nucleus! ❌ But atoms are stable, proving Rutherford’s model incomplete.
2️⃣ Did Not Explain Energy Levels 🔋💡
📌 Rutherford’s model did not explain why electrons stay in fixed orbits instead of continuously losing energy. Bohr’s model later introduced energy levels 🔄➰.
3️⃣ Failed to Describe Atomic Spectrum 🌈⚛️
📌 Rutherford’s model did not explain why atoms emit specific spectral lines when excited! 🌟 Bohr’s theory later solved this using quantized energy levels.
🎯 Board Exam Tip:
✅ Mention Rutherford’s gold foil experiment 📜.
✅ Clearly explain why electrons should collapse but don’t 🔬.
✅ Relate it to Bohr’s model improvements 🚀.
✅ Keep your answer well-structured & precise for full marks 💯🏆!
Q. 4. Describe Bohr’s model of the atom.
Answer:-
✨ Bohr’s Model of the Atom ⚛️🔬
Niels Bohr proposed an improved atomic model 🌟 that explained how electrons behave inside an atom!
🔹 Key Features of Bohr’s Model ⚛️💡
1️⃣ Electrons Revolve in Fixed Orbits 🔄➰
📌 Electrons ⚡ move around the nucleus 🏡 in specific circular paths (shells) instead of randomly!
2️⃣ Energy Levels are Quantized 🔋✨
📌 Electrons stay in fixed energy levels 🌀 and do not lose energy while revolving!
3️⃣ Electrons Jump Between Energy Levels ⚡📊
📌 Electrons can absorb energy 🔥 and move to a higher level, or release energy 🌈 to return to a lower level!
🔹 Example: Bohr’s Model of Hydrogen (H) 🌬️
✅ Single electron ⚡ revolves around the nucleus 🏡 in a defined orbit 🔄.
✅ The energy of the electron depends on its shell position!
🎯 Board Exam Tip:
✅ Mention that Bohr’s model improved Rutherford’s theory.
✅ Explain energy levels, electron movement & stability.
✅ Draw a simple labeled diagram ✏️📜 for visual clarity!
✅ Keep answers neat & structured to secure full marks 💯🏆!
Q. 5. Compare all the proposed models of an atom given in this chapter.
Answer:-
✨ Comparison of All Proposed Atomic Models ⚛️🔬
Over time, scientists developed different models to explain the structure of an atom 🌍⚡. Each model had strengths and limitations that helped refine our understanding! 🚀
📜 Tabular Comparison of Atomic Models
| Model 🔬 | Scientist 🏆 | Key Features 💡 | Limitations ❌ |
|---|---|---|---|
| Dalton’s Atomic Model ⚛️ | John Dalton (1808) | 🌟 Atoms are indivisible and cannot be created or destroyed. | ❌ Could not explain sub-atomic particles and atomic structure. |
| Thomson’s Model (Plum Pudding) 🍮 | J.J. Thomson (1897) | 🌟 Atoms are positively charged spheres ⚡ with embedded electrons ⚫. | ❌ Failed to explain the existence of a nucleus and atomic stability. |
| Rutherford’s Model 🔬✨ | Ernest Rutherford (1911) | 🌟 Atoms have a dense, positively charged nucleus 🏡 with electrons revolving around it. | ❌ Could not explain why electrons don’t lose energy and collapse into the nucleus. |
| Bohr’s Model 🔄➰ | Niels Bohr (1913) | 🌟 Electrons move in fixed energy levels 🔋 and absorb/release energy when changing levels. | ❌ Could not explain subatomic interactions & wave behavior of electrons. |
| Quantum Mechanical Model 📊⚛️ | Schrödinger & Heisenberg (1926) | 🌟 Electrons exist in probability clouds (orbitals) rather than fixed paths. | ❌ Complex model, difficult to visualize atomic structure easily. |
🎯 Board Exam Tip:
✅ Use structured tables 📊 for better clarity!
✅ Mention scientists & key discoveries 🏆.
✅ Explain how models evolved and their limitations.
✅ Keep answers well-organized to ensure full marks 💯🏆!
Q. 6. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer:-
✨ Rules for Electron Distribution in Various Shells ⚛️🔬
Electrons ⚡ in an atom are arranged in shells (energy levels) 🔄➰ around the nucleus 🏡. The distribution follows specific rules to determine the electronic configuration of an element.
🔹 Rules for Electron Distribution 📜💡
1️⃣ Maximum Electrons in a Shell 🔋✨
📌 The maximum number of electrons that a shell can hold is given by the formula:
2n² (where n is the shell number)
✅ K-shell (n = 1) ➡ 2 × (1²) = 2 electrons ⚡⚡
✅ L-shell (n = 2) ➡ 2 × (2²) = 8 electrons ⚡⚡⚡⚡⚡⚡⚡⚡
✅ M-shell (n = 3) ➡ 2 × (3²) = 18 electrons ⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡⚡
2️⃣ Outer Shell Cannot Hold More than 8 Electrons ⚖️🔬
📌 Even though the M-shell can hold 18 electrons, it cannot have more than 8 electrons unless the next shell starts filling!
3️⃣ Filling Order of Shells 🔄➰
📌 Electrons fill shells in order of increasing energy levels:
K → L → M → N 🔄
🔹 Example: Electron Distribution for First 18 Elements 🧪⚛️
| Element 🏗️ | Atomic Number 🔢 | Electron Configuration ⚡ |
|---|---|---|
| Hydrogen (H) 🌬️ | 1 | 1 |
| Helium (He) 💨 | 2 | 2 |
| Lithium (Li) 🔋 | 3 | 2, 1 |
| Carbon (C) 🏗️ | 6 | 2, 4 |
| Oxygen (O) 🌬️ | 8 | 2, 6 |
| Sodium (Na) 🏗️ | 11 | 2, 8, 1 |
| Chlorine (Cl) 💡 | 17 | 2, 8, 7 |
| Argon (Ar) 🌍 | 18 | 2, 8, 8 |
🎯 Board Exam Tip:
✅ Use formula 2n² to show maximum electrons in each shell!
✅ Clearly define rules for electron filling.
✅ Draw a labeled diagram ✏️📜 of electron distribution!
✅ Keep answers structured & precise to secure full marks 💯🏆!
Q. 7. Define valency by taking examples of silicon and oxygen.
Answer:-
✨ Definition of Valency with Examples of Silicon & Oxygen ⚛️🔬
Valency refers to the combining capacity of an atom 🌍🔗. It is determined by the number of electrons present in the outermost shell 🔄➰ of an atom.
🔹 Valency of Silicon (Si) 🏗️⚛️
🔸 Atomic Number = 14 🔢
🔸 Electronic Configuration = 2, 8, 4 ⚡⚡➰➰➰➰
📌 Valency = 4 ➕✨
✅ Silicon needs 4 more electrons to complete its octet, making its valency 4!
💡 Example: Si forms bonds with oxygen in silica (SiO₂)! 🔬
🔹 Valency of Oxygen (O) 🌬️⚛️
🔸 Atomic Number = 8 🔢
🔸 Electronic Configuration = 2, 6 ⚡⚡➰➰➰➰➰➰
📌 Valency = 2 ➕✨
✅ Oxygen needs 2 more electrons to complete its octet, making its valency 2!
💡 Example: Oxygen forms bonds in water (H₂O) and carbon dioxide (CO₂)! 💧🌍
🎯 Board Exam Tip:
✅ Clearly define valency in simple terms.
✅ Show electron configurations to explain how valency is determined.
✅ Use real-life examples of compounds!
✅ Keep answers structured & precise to secure full marks 💯🏆!
Hope this helps, Anurag! 🚀✨ Let me know if you need refinements! 😊⚛️🔬
Q. 8. Explain with examples (i) Atomic number, (ii) Mass number, (iii) Isotopes and iv) Isobars. Give any two uses of isotopes.
Answer:-
✨ Explanation with Examples: Atomic Number, Mass Number, Isotopes & Isobars ⚛️🔬
Atoms are made up of protons, neutrons, and electrons 🏡. Their characteristics are defined using atomic number, mass number, isotopes, and isobars! 🚀
(i) Atomic Number (Z) 🔢⚛️
📌 Definition: The atomic number of an element is the number of protons 🔋 in its nucleus 🏡.
📌 Formula: Atomic Number (Z) = Number of Protons
📌 Example:
✅ Hydrogen (H) 🌬️ has 1 proton, so its atomic number = 1.
✅ Carbon (C) 🏗️ has 6 protons, so its atomic number = 6.
(ii) Mass Number (A) ⚖️💡
📌 Definition: The mass number of an atom is the sum of protons and neutrons present in the nucleus 🏡.
📌 Formula: Mass Number (A) = Number of Protons + Number of Neutrons
📌 Example:
✅ Oxygen (O) 🌬️ has 8 protons and 8 neutrons, so its mass number = 16.
✅ Sulphur (S) 🏗️ has 16 protons and 16 neutrons, so its mass number = 32.
(iii) Isotopes ⚛️🔬
📌 Definition: Isotopes are atoms of the same element that have the same atomic number but different mass numbers! 🔄
📌 Example:
✅ Hydrogen Isotopes 🌬️:
- Protium (¹H): 1 proton, 0 neutrons, mass number = 1.
- Deuterium (²H): 1 proton, 1 neutron, mass number = 2.
- Tritium (³H): 1 proton, 2 neutrons, mass number = 3.
(iv) Isobars ⚖️✨
📌 Definition: Isobars are atoms of different elements that have the same mass number but different atomic numbers! 🌍
📌 Example:
✅ Calcium-40 (⁴⁰Ca) & Argon-40 (⁴⁰Ar):
- Calcium (Ca) ➡ Atomic Number = 20 🔢, Mass Number = 40.
- Argon (Ar) ➡ Atomic Number = 18 🔢, Mass Number = 40.
Uses of Isotopes 🔬💡
📌 1️⃣ Medical Applications 🏥
✅ Cobalt-60 (⁶⁰Co) is used in cancer treatment (radiotherapy)!
✅ Iodine-131 (¹³¹I) is used to treat thyroid disorders!
📌 2️⃣ Industrial & Scientific Uses 🏗️
✅ Carbon-14 (¹⁴C) is used in carbon dating to determine the age of fossils!
✅ Uranium-235 (²³⁵U) is used in nuclear power plants!
🎯 Board Exam Tip:
✅ Clearly define each term and provide examples.
✅ Show calculations for atomic & mass number.
✅ Use a structured format to make answers clear & engaging!
✅ Diagrams & tables 📜📊 can add visual appeal!
Q. 9. Na+ has completely filled K and L shells. Explain.
Answer:-
✨ Explanation: Na⁺ has Completely Filled K and L Shells ⚛️🔬
Sodium (Na) 🏗️ has an atomic number of 11 🔢, meaning it has 11 electrons ⚡ in its neutral state.
✅ Electronic Configuration of Neutral Na: 2, 8, 1 ➰➰➰
🔹 Formation of Na⁺ Ion ⚡✨
- Sodium loses 1 electron ⚡ from its M-shell (third shell).
- This happens because atoms tend to achieve a stable electronic configuration (like noble gases 🌍💡).
- After losing one electron, Na⁺ has only 10 electrons left! ✅
📌 New Electronic Configuration of Na⁺: 2, 8 ➰➰
✅ Now, only K and L shells are occupied and they are completely filled! 🏆✨
🎯 Board Exam Tip:
✅ Clearly explain how Na⁺ is formed.
✅ Mention the stable configuration goal.
✅ Use a neat diagram ✏️📜 to show electron distribution!
✅ Keep explanations structured & precise for full marks 💯🏆!
Q. 10. If bromine atom is available in the form of, say, two isotopes 7935Br (49.7%) and 8135 Br (50.3%), calculate the average atomic mass of bromine atom.
Answer:-
✨ Calculation of Average Atomic Mass of Bromine ⚛️🔬
A bromine atom exists in two isotopic forms:
🔹 79Br (Atomic Mass = 79 u, Abundance = 49.7%)
🔹 81Br (Atomic Mass = 81 u, Abundance = 50.3%)
🧮 Formula for Average Atomic Mass ⚖️💡
📌 Average Atomic Mass =
[ \frac{(Mass \times Abundance) + (Mass \times Abundance)}{100} ]
📊 Substituting Values
📌 Average Atomic Mass =
[ (79 x 49.7) + (81 x 50.3) ]/100
📌 Average Atomic Mass =
[ 3936.3 + 4084.3 x100 ] = [8020.6/100] = 80.2 u
✅ Final Answer:
The average atomic mass of bromine = 80.2 u ⚖️✨
🎯 Board Exam Tip:
✅ Clearly show step-by-step calculations 🧮.
✅ Define the formula and concept of average atomic mass.
✅ Keep answers neat, structured & well-explained to secure full marks 💯🏆!
Q. 11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168 X and 18 8 X in the sample?
Answer:-
✨ Calculation of Percentage of Isotopes ⚛️🔬
Given:
🔹 Average Atomic Mass of X = 16.2 u ⚖️
🔹 Isotopes Present:
- (168X) (Atomic Mass = 16 u)
- (188X) (Atomic Mass = 18 u)
🧮 Formula for Percentage of Isotopes ⚖️💡
Let x% be the percentage of (168X) and (100 – x)% be the percentage of (188X).
📌 Using weighted average formula:
[ (16(x) + (18 x (100 – x)) = 16.2 x 100 ]
📊 Substituting Values & Solving
[ 16x + 1800 – 18x = 1620 ]
[ -2x + 1800 = 1620 ]
[ -2x = -180 ]
[ x = 90 ]
📌 Percentage of (168X) = 90%
📌 Percentage of (188X) = 100 – 90 = 10%
✅ Final Answer:
🔹 90% of the sample consists of (168X) ⚛️✅
🔹 10% of the sample consists of (188X) ⚛️✅
🎯 Board Exam Tip:
✅ Show step-by-step calculations clearly 🧮.
✅ Define weighted atomic mass formula 📜✨.
✅ Keep answers structured & precise for full marks 💯🏆!
Q. 12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer:-
✨ Valency and Name of the Element for Z = 3 ⚛️🔬
The atomic number (Z) represents the number of protons 🔋 in an atom.
📌 Given Z = 3, this means the element has 3 protons, so it is Lithium (Li) 🏗️⚛️!
🔹 Electronic Configuration of Lithium 🧮
🔸 Atomic Number = 3 🔢
🔸 Electronic Configuration = 2, 1 ⚡⚡➰
🔹 Determining Valency ✨
📌 Valency = Number of electrons in the outermost shell 🔄➰
✅ Lithium has 1 electron in its outermost shell, so its valency = 1! ➕✨
✅ Final Answer:
🔹 The element is Lithium (Li) ⚛️🏗️.
🔹 Valency of Lithium = 1! ✨
🎯 Board Exam Tip:
✅ Define atomic number & valency 📜.
✅ Show electronic configuration & how valency is determined.
✅ Keep answers structured & precise for full marks 💯🏆!
Q. 13. Composition of the nuclei of two atomic species X and Y are given as under
| X | Y | |
| Protons | 6 | 6 |
| Neutrons | 6 | 8 |
Give the mass numbers of X and Y. What is the relation between the two species?
Answer:-
✨ Mass Number Calculation & Relation Between Species ✨
Let’s analyze atomic species X and Y based on their nuclear composition:
| Atomic Species | Protons (𝑍) | Neutrons (𝑁) | Mass Number (𝐴 = 𝑍 + 𝑁) |
|---|---|---|---|
| X | 6️⃣ | 6️⃣ | 1️⃣2️⃣ |
| Y | 6️⃣ | 8️⃣ | 1️⃣4️⃣ |
📌 Mass Number Calculation:
Mass number (𝐴) is determined by the sum of protons and neutrons:
[ 𝐴 = 𝑍 + 𝑁 ]
For X:
🔹 𝐴 = 6 + 6 = 12
For Y:
🔹 𝐴 = 6 + 8 = 14
📌 Relation Between X and Y:
Since both species have the same number of protons (𝑍 = 6) but different numbers of neutrons, they are isotopes of the same element.
🧪 Definition of Isotopes:
👉 Isotopes are atomic species with the same atomic number (𝑍) but different neutron numbers.
🚀 Final Answer for Full Marks:
✔ X and Y have mass numbers 12 and 14, respectively.
✔ X and Y are isotopes because they belong to the same element (𝑍 = 6) but differ in neutron count.
Q. 14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:-
✨ True & False Statements – Answer with Explanation ✨
Here’s the correct evaluation of the statements for your Class 9 board exams! 🎯
| Statement | True / False | Explanation |
|---|---|---|
| (a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. | ❌ False | J.J. Thomson proposed the plum pudding model, where electrons were embedded in a positive sphere. The concept of nucleons (protons & neutrons) in the nucleus was introduced later by Rutherford & Chadwick. |
| (b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. | ❌ False | A neutron is not formed by the combination of an electron and a proton. It is a fundamental subatomic particle present in the nucleus with zero charge. |
| (c) The mass of an electron is about 1/2000 times that of a proton. | ✅ True | The mass of an electron is approximately 1/1836 times that of a proton, which is close to 1/2000, hence this statement is considered true for approximation purposes. ⚖️ |
| (d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. | ❌ False | Tincture iodine (used as an antiseptic) contains iodine dissolved in alcohol, but radioactive isotopes of iodine (like I-131) are used in medical treatments (not in tincture iodine). 💊 |
🚀 Final Answer for Full Marks:
✔ (a) False
✔ (b) False
✔ (c) True
✔ (d) False
Put tick (¸) against correct choice and cross (×) against wrong choice in questions 15, 16 and 17
Q. 15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
Answer:-
✨ Rutherford’s Alpha-Particle Scattering Experiment – Discovery ✨
🚀 Question: Rutherford’s alpha-particle scattering experiment was responsible for the discovery of:
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
✅ Correct Answer: (a) Atomic Nucleus
📌 Explanation:
🔬 Rutherford’s gold foil experiment demonstrated that:
✔ Atoms have a small, dense, positively charged nucleus at their center.
✔ Most of the atom’s space is empty, allowing alpha particles to pass through without deflection.
✔ Some alpha particles deflected at large angles, proving the presence of a central nucleus that repelled them.
📢 Why Not Others?
❌ Electron: Discovered by J.J. Thomson in his cathode ray experiment.
❌ Proton: Rutherford later proposed the proton’s existence, but his experiment directly led to the nucleus discovery.
❌ Neutron: Discovered later by James Chadwick in 1932.
🚀 Final Answer for Full Marks:
✔ Rutherford’s experiment led to the discovery of the atomic nucleus.
✔ Correct choice: (a) Atomic Nucleus.
Q. 16. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers.
Answer:-
✨ Understanding Isotopes – Answer & Explanation ✨
🚀 Question: Isotopes of an element have:
(a) The same physical properties
(b) Different chemical properties
(c) Different number of neutrons
(d) Different atomic numbers
✅ Correct Answer: (c) Different number of neutrons
📌 Explanation:
🧪 Isotopes are atoms of the same element that have:
✔ Same atomic number (𝑍) → Same number of protons
✔ Different number of neutrons → Different mass numbers
📢 Why Not Others?
❌ (a) Same physical properties:
👉 Some physical properties do vary, especially those related to mass (like density & boiling point). So this is incorrect.
❌ (b) Different chemical properties:
👉 Isotopes have the same number of electrons and protons, so their chemical properties remain the same.
❌ (d) Different atomic numbers:
👉 Atomic number is always the same for isotopes of an element. If atomic number changes, it becomes a different element, not an isotope.
🚀 Final Answer for Full Marks:
✔ Isotopes of an element have different numbers of neutrons.
✔ Correct choice: (c) Different number of neutrons.
Q. 17. Number of valence electrons in Cl– ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer:-
✨ Valence Electrons in Cl⁻ Ion – Answer & Explanation ✨
🚀 Question: The number of valence electrons in Cl⁻ ion are:
(a) 16
(b) 8
(c) 17
(d) 18
✅ Correct Answer: (b) 8
📌 Explanation:
🧪 Chlorine (Cl) Atomic Number = 17
✔ In a neutral chlorine atom, the electron configuration is 2, 8, 7 → 7 valence electrons.
✔ When chlorine gains 1 electron to form Cl⁻ ion, it has 8 valence electrons (like noble gas configuration).
📢 Why Not Others?
❌ (a) 16: Total electrons in Cl⁻ are 18, but valence electrons refer to only outermost shell electrons.
❌ (c) 17: Chlorine has 17 protons, but the number of electrons in the valence shell changes when forming Cl⁻.
❌ (d) 18: Total electrons in Cl⁻ ion = 18, but valence electrons = 8.
🚀 Final Answer for Full Marks:
✔ Cl⁻ ion has 8 valence electrons.
✔ Correct choice: (b) 8.
Q. 18. 🚀 Question: Which one of the following is the correct electronic configuration of sodium (Na)?
(a) 2,8
(b) 8,2,1
(c) 2,1,8
(d) 2,8,1
Answer:-
✨ Electronic Configuration of Sodium (Na) – Answer & Explanation ✨
✅ Correct Answer: (d) 2,8,1
📌 Explanation:
🧪 Sodium (Na) Atomic Number = 11
✔ The atomic number represents the total number of electrons in a neutral atom of sodium.
✔ Electrons are arranged in shells following the 2, 8, 18 rule for distribution.
🔹 Step-by-Step Electron Distribution:
1️⃣ First shell (K): Max capacity = 2 electrons → 2
2️⃣ Second shell (L): Max capacity = 8 electrons → 8
3️⃣ Third shell (M): Remaining 1 electron → 1
📢 Why Not Others?
❌ (a) 2,8: Incorrect because sodium has 11 electrons, not just 10.
❌ (b) 8,2,1: Incorrect as electron filling follows 2,8,1 order, not 8 first.
❌ (c) 2,1,8: Incorrect as electrons follow K → L → M shell filling rule.
🚀 Final Answer for Full Marks:
✔ Sodium’s electronic configuration is 2,8,1.
✔ Correct choice: (d) 2,8,1.
Q. 19. Complete the following table.
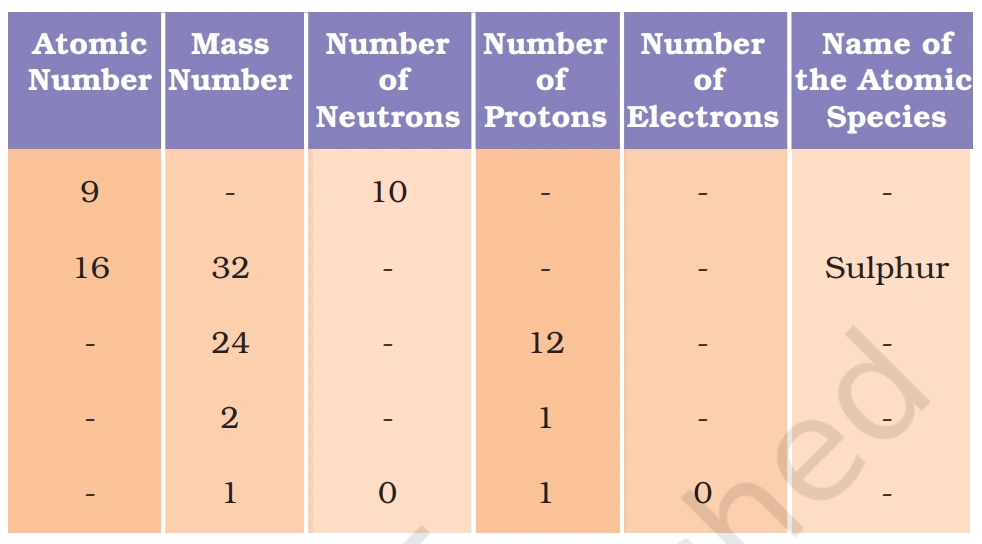
Answer:-
Here’s the completed table with all missing values filled in for clarity! ✨
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
|---|---|---|---|---|---|
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulphur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 0 | 1 | 1 | Hydrogen |
📢 How was the table completed?
✔ Number of neutrons = Mass Number – Atomic Number
✔ Number of protons = Atomic Number
✔ Number of electrons = Atomic Number (for neutral atoms)