NCERT Science Class 9 Chapter 1 Question Answer – Matter in Our Surroundings FREE PDF Download
Class 9 Science – Chapter 1: Matter in Our Surroundings
In Chapter 1: Matter in Our Surroundings, we explore the fundamental principles of matter and its different states—solid, liquid, and gas. This chapter provides a deep understanding of the properties of matter, the changes it undergoes, and key concepts like evaporation.
By studying these concepts, students build a strong foundation for grasping how matter behaves in various conditions. With clear explanations and structured solutions, Class 9 Science solutions make learning engaging and accessible.
Let’s dive in and uncover the fascinating world of matter in our surroundings! 🚀
Let me know if you need further refinements! 😊
Get your FREE PDF of NCERT Solutions for Class 9 Science Chapter 1, fully updated as per the latest syllabus! Strengthen your understanding of Matter in Our Surroundings and boost your academic success with cbseprep.in.
Start your learning journey today! 🚀
Let me know if you need any refinements. 😊
Quick Insights into Matter in Our Surroundings – NCERT Solutions for Class 9 Science
Explore a comprehensive overview of the states of matter and their significance in daily life.
Master the key concepts of solids, liquids, and gases, which form the foundation for understanding material properties and phase transitions.
In Chapter 1 of Class 9 Science, delve into the characteristics of matter, including intermolecular forces, kinetic theory, and the impact of temperature on state changes.
The NCERT Solutions for Class 9 Science Chapter 1 provide clear and detailed explanations, ensuring complete exam preparation. Strengthen your ability to solve numerical problems related to the behavior of matter across different states, enhancing your problem-solving skills.
CbsePrep.in offers valuable learning resources, including class notes, important concepts, formulas, and exemplar solutions, helping students build a strong foundation in scientific principles.
NCERT Textbook for Class 9 Science – Page 3
Q. 1. Which of the following are the matter?
Answer:-
Matter and Its Classification
Matter is defined as anything that has mass and occupies space. It exists in three fundamental states: solid, liquid, and gas.
Classification of Matter Based on Its States
1. Solid State:
- Solids have a fixed shape and definite volume.
- Example: Chair and almond —these objects maintain their shape and do not flow.
2. Liquid State:
- Liquids have a definite volume but no fixed shape; they take the shape of the container and can flow.
- Example: Cold drink —it conforms to the container and exhibits fluidity.
3. Gaseous State:
- Gases neither have a fixed shape nor a definite volume. Their particles move freely in all directions.
- Example: Air and the smell of perfume—both spread in all directions due to high kinetic energy of gas particles.
Important Note: What Is NOT Matter?
Certain entities such as love, hate, cold, smell, and thoughts do not possess mass or occupy space. These are sensations or emotions experienced by human beings and hence, do not qualify as matter.
Q. 2 Give reasons for the following observation:
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Answer:-
Diffusion and Temperature Dependence
The phenomenon where the smell of hot sizzling food prepared in the kitchen reaches a distant room but diminishes as the food cools down can be explained by the rate of diffusion and its dependence on temperature.
Explanation of Diffusion in Hot and Cold Food
- Diffusion is the process by which particles spread out from a region of higher concentration to a region of lower concentration.
- The rate of diffusion increases with temperature, as higher temperatures impart more kinetic energy to the particles, enabling them to move faster and mix with air more efficiently.
Why Hot Food Smells Stronger than Cold Food
- Hot food has higher temperature, meaning its molecules possess greater kinetic energy and diffuse rapidly into the air.
- Cold food has lower temperature, causing reduced molecular movement, hence slower diffusion, making the smell less perceptible.
Conclusion
Since temperature directly affects molecular motion, diffusion of food aroma is more effective at higher temperatures, explaining why the smell of hot food spreads widely but diminishes once the food cools down.
Q. 3 A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer:-
Property of Matter Demonstrated by a Diver Cutting Through Water
The ability of a diver to cut through water in a swimming pool illustrates the property of fluidity and weak intermolecular forces in liquids.
Explanation:
1. Fluidity of Liquids
- Liquids do not have a fixed shape and can flow easily.
- Water allows objects, like a diver, to move through it because its particles are loosely packed and can shift their positions.
2. Weak Intermolecular Forces
- In liquids, the intermolecular forces are weaker than in solids, enabling free movement of molecules.
- A diver can pass through water because water molecules can move aside to make space.
Conclusion
The ability of a diver to move through water is a result of the fluidity and weak intermolecular forces in liquids. These properties allow liquids to flow and adapt to the shape of their container.
Q. 4: What are the characteristics of particles of matter?
Answer:-
Characteristics of Particles of Matter
The fundamental characteristics of particles of matter are as follows:
1. Matter is Made Up of Tiny Particles
- All matter consists of extremely small particles that may not be visible to the naked eye.
- Example: Sugar dissolves in water, but its particles remain present even though they become invisible.
2. Particles of Matter Have Space Between Them
- The particles of matter are not tightly packed; there is always some space between them.
- Example: When salt or sugar is added to water, it dissolves without increasing the water level, indicating the presence of spaces between water molecules.
3. Particles of Matter Are Constantly Moving
- The particles of matter are always in motion, and their movement increases with temperature.
- Example: The smell of perfume spreads quickly due to the movement of gaseous particles.
4. Particles of Matter Attract Each Other
- The particles of matter exert a force of attraction, which is strongest in solids, moderate in liquids, and weakest in gases.
- Example: It is harder to break a solid object like a piece of iron due to strong intermolecular forces, whereas gas particles move freely with minimal attraction.
NCERT Textbook for Class 9 Science – Page 6
Q. 1 The mass per unit volume of a substance is called density (density = mass/volume). Arrange the following in order of increasing density – air, exhaust from chimney, honey, water, chalk, cotton, and iron.
Answer:-
Density and Its Increasing Order
Density is defined as the mass per unit volume of a substance, expressed as:
Density = Mass\Volume
Arranging the Given Substances in Increasing Density Order
The substances can be arranged from lowest to highest density based on their physical properties:
- Air – Being a gas, air has the least density among the listed substances.
- Exhaust from Chimney – This is also a gas but contains heavier particles than air, slightly increasing its density.
- Cotton – Although cotton appears solid, it has a lot of air trapped between fibers, making it less dense than most solids.
- Water – A liquid with moderate density, higher than gases but lower than solids.
- Honey – A dense liquid with greater density than water due to its thicker consistency.
- Chalk – A solid but still porous, making it less dense than iron.
- Iron – A compact solid with maximum density among the given substances.
Final Order of Increasing Density:
➡ Air < Exhaust from Chimney < Cotton < Water < Honey < Chalk < Iron
Q. 2(a) tabulate the differences in the characteristics of states of matter.
Answer:-
Differences in the Characteristics of States of Matter
The three primary states of matter—solid, liquid, and gas—differ based on several properties. The table below summarizes these differences:
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Fixed | No fixed shape, takes the shape of the container | No fixed shape, expands to fill available space |
| Volume | Fixed | Fixed | No fixed volume, occupies the entire container |
| Arrangement of Particles | Tightly packed | Loosely packed | Very far apart, free movement |
| Intermolecular Forces | Strong | Moderate | Weak |
| Compressibility | Negligible | Slightly compressible | Highly compressible |
| Fluidity | Does not flow | Flows freely | Flows and spreads in all directions |
| Diffusion | Extremely slow or negligible | Faster than solids | Rapid diffusion |
| Kinetic Energy | Lowest among the three states | Moderate | Highest among the three states |
Q. 2 (b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Answer:-
Commentary on the Given Properties of Matter
Each property describes a fundamental characteristic that helps distinguish the three states of matter—solid, liquid, and gas.
1. Rigidity
- Rigidity refers to the ability of a substance to retain its shape and resist deformation.
- Solids are rigid because their particles are closely packed and held by strong intermolecular forces.
- Example: A stone does not change shape when placed in different containers.
2. Compressibility
- Compressibility is the ability of a substance to reduce in volume under applied pressure.
- Gases are highly compressible, whereas solids and liquids show negligible compression.
- Example: A gas cylinder contains compressed oxygen, which occupies much less space than in its free form.
3. Fluidity
- Fluidity refers to the ability of a substance to flow and take the shape of the container.
- Liquids and gases exhibit fluidity, but solids do not due to their fixed shape.
- Example: Water poured into different containers takes their shape.
4. Filling a Gas Container
- Gases completely fill the container they are placed in due to their free-moving particles.
- They do not have a definite shape or volume but expand to occupy available space.
- Example: Air inside a balloon fills up completely, taking its shape.
5. Shape
- Solids have a definite shape due to strong intermolecular forces.
- Liquids take the shape of the container they are poured into.
- Gases spread throughout the space available.
- Example: A wooden cube retains its shape, while water changes shape based on the vessel.
6. Kinetic Energy
- Kinetic energy refers to the energy possessed by moving particles.
- Gaseous particles have the highest kinetic energy, followed by liquids and then solids.
- Example: Steam particles move rapidly, making gas spread quickly.
7. Density
- Density is defined as the mass per unit volume of a substance:
Density = Mass/Volume - Solids usually have the highest density, liquids are moderately dense, and gases have the lowest density.
- Example: Iron is denser than water, so it sinks, while oil is less dense than water, so it floats.
This structured explanation aligns with CBSE Class 9 board examination standards, ensuring clarity with examples. Let me know if you need any refinements!
Q. 3 Give Reasons:
(a) A gas fills completely the vessel in which it is kept.
Answer:-
Why Do Gases Completely Fill the Vessel They Are Kept In?
The ability of gases to completely fill the container in which they are placed is due to their unique properties.
Explanation Based on Gas Properties:
- High Kinetic Energy
- Gas particles move randomly and at high speeds in all directions.
- Due to their high kinetic energy, they spread out and fill the entire available space.
- Weak Intermolecular Forces
- The force of attraction between gas molecules is very weak or negligible.
- This allows gas particles to move freely without being bound together.
- Indefinite Shape and Volume
- Unlike solids and liquids, gases do not have a fixed shape or volume.
- They take the shape and volume of the container they are placed in.
Example:
- Air inside a balloon spreads uniformly, taking its shape.
- Cooking gas (LPG) expands to completely fill the cylinder.
Conclusion:
Gases expand freely because of their high kinetic energy, weak intermolecular forces, and indefinite shape and volume, ensuring they fill the entire vessel in which they are kept.
(b) A gas exerts pressure on the walls of the container.
Answer:-
Why Do Gases Exert Pressure on the Walls of a Container?
Gases exert pressure on the walls of the container due to the constant motion and collision of gas particles.
Explanation Based on Gas Properties:
- Random Motion of Gas Molecules
- Gas particles move continuously and randomly in all directions.
- This motion causes frequent collisions with the walls of the container.
- Force Due to Molecular Collisions
- Each time a gas molecule collides with the container’s walls, it exerts a force.
- The total force per unit area exerted by all molecules is called gas pressure.
- Effect of Temperature on Pressure
- Increasing temperature raises kinetic energy, causing molecules to collide more frequently and exert higher pressure.
- Example: A bursting balloon when overheated due to excessive gas pressure inside.
Example:
- Tire pressure increases when air is pumped in because gas molecules collide with the tire’s inner walls.
- An inflated football remains firm due to gas pressure inside pushing outward.
Conclusion:
Gases exert pressure due to molecular collisions against the container walls. Higher temperature or more gas particles lead to greater pressure inside a closed system.
(c) A wooden table should be called a solid.
Answer:-
Why Is a Wooden Table Considered a Solid?
A wooden table is classified as a solid because it possesses the fundamental characteristics of solids.
Characteristics That Make a Wooden Table a Solid:
- Definite Shape and Volume
- A wooden table has a fixed shape and occupies a definite volume that does not change on its own.
- Rigid Structure
- The particles in a wooden table are tightly packed with strong intermolecular forces, making it rigid and hard.
- Incompressibility
- Unlike gases or some liquids, a wooden table cannot be compressed easily due to its tightly bound molecules.
- Non-Fluidity
- Solids do not flow like liquids or gases, and a wooden table retains its form irrespective of its surroundings.
Conclusion:
A wooden table is considered a solid because it has a fixed shape, definite volume, strong intermolecular forces, rigidity, and lacks fluidity, making it distinct from liquids and gases.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Answer:-
Why Is It Easy to Move a Hand in Air but Difficult Through a Solid Block of Wood?
This difference is due to the nature of intermolecular forces and particle arrangement in different states of matter.
1. Intermolecular Forces
- Air is a gas, with very weak intermolecular forces, allowing particles to move freely in all directions.
- Wood is a solid, with strong intermolecular forces, making its particles tightly packed and rigid.
2. Particle Arrangement
- In gases (like air), molecules are spread far apart, creating little resistance when moving a hand.
- In solids (like wood), molecules are closely packed, making movement difficult without applying force.
3. Compressibility
- Air is highly compressible, meaning its molecules can shift easily, letting a hand pass through without resistance.
- Wood is an incompressible solid, requiring strong force, such as that applied by a karate expert, to break through.
Conclusion
The ease of moving a hand in air compared to a solid block of wood is due to weaker intermolecular forces, greater molecular spacing, and compressibility of gases, whereas solids have strong intermolecular forces, tightly packed molecules, and rigidity.
Q. 4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out Why.
Answer:-
Why Does Ice Float on Water Despite Being a Solid?
It is generally observed that solids have higher density than liquids, but ice floats on water due to a unique property of water when it freezes.
Reason: Density Anomaly of Water
- Expansion of Water Upon Freezing
- When water freezes, it expands instead of contracting.
- This expansion increases volume, thereby reducing the density of ice compared to liquid water.
- Density of Ice vs. Water
- The density of liquid water is approximately 1 g/cm³, whereas ice has a density of about 0.92 g/cm³.
- Since ice has a lower density than water, it floats on the surface instead of sinking.
- Structure of Ice Molecules
- In liquid water, molecules move freely and are closely packed.
- In ice, water molecules arrange in a hexagonal crystal structure with gaps between them, making ice less dense than liquid water.
Example in Nature
- Floating icebergs in oceans allow marine life to thrive underneath.
- Frozen lakes have ice on the surface, while liquid water remains below, protecting aquatic life during winters.
Conclusion
Ice floats on water because it is less dense than liquid water due to molecular expansion upon freezing. This density anomaly of water makes it an exception to the usual solid-liquid density relationship.
NCERT Textbook for Class 9 Science – Page 9
Q. 1 Convert the following temperature to Celsius scale:
(a) 300 K
(b) 573 K
Answer:-
Conversion of Temperature from Kelvin to Celsius
Temperature can be converted from Kelvin (K) to Celsius (°C) using the formula:
Celsius temperature = Kelvin temperature – 273
Given Temperatures:
(a) 300 K to Celsius
[ 300 – 273 = 27°C ]
(b) 573 K to Celsius
[ 573 – 273 = 300°C ]
Final Answer:
- 300 K = 27°C
- 573 K = 300°C
Q. 2. What is the physical state of water at:
(a) 250o C
(b) 100o C
Answer:-
Physical State of Water at Different Temperatures
The physical state of water depends on temperature and pressure. Under standard atmospheric pressure (1 atm or 101.3 kPa), the states of water at the given temperatures are:
(a) At 250°C
- Water exists in the gaseous state as steam or water vapor.
- This is because the boiling point of water is 100°C under normal atmospheric pressure, and at 250°C, it remains in vapor form.
(b) At 100°C
- Water is in a transition state between liquid and gas.
- If heat is continuously supplied, water changes from liquid to gaseous state (steam) due to boiling.
- If pressure increases above 1 atm, water may remain in the liquid state even at 100°C.
Conclusion
- At 250°C → Water exists as gas (steam/vapor).
- At 100°C → Water transitions from liquid to gas under normal atmospheric pressure.
Q. 3. For any substance, why does the temperature remain constant during the change of state?
Answer:-
Why Does Temperature Remain Constant During a Change of State?
When a substance undergoes a change of state (such as melting, boiling, or condensation), its temperature remains constant despite continuous heat supply. This phenomenon occurs due to latent heat.
Explanation: Role of Latent Heat
- Energy Used for Phase Change
- During melting, boiling, or condensation, the heat energy supplied does not increase temperature.
- Instead, this energy is used to break or form intermolecular bonds, changing the state of the substance.
- Latent Heat Definition
- The energy required to change the state of a substance without changing temperature is called latent heat.
- Latent heat of fusion is used when a solid changes to liquid.
- Latent heat of vaporization is used when a liquid changes to gas.
Example:
- When ice melts at 0°C, it absorbs heat but remains at 0°C until completely melted.
- When water boils at 100°C, it continues to absorb heat without increasing temperature, transforming into steam.
Conclusion:
Temperature remains constant during a change of state because the absorbed or released heat energy is used to alter intermolecular bonding, rather than raising temperature. This ensures a smooth transition between states.
Q. 3. For any substance, why does the temperature remain constant during the change of state?
Answer:-
Why Does Temperature Remain Constant During a Change of State?
When a substance undergoes a change of state (such as melting, boiling, or condensation), its temperature remains constant despite continuous heat supply. This phenomenon occurs due to latent heat.
Explanation: Role of Latent Heat
- Energy Used for Phase Change
- During melting, boiling, or condensation, the heat energy supplied does not increase temperature.
- Instead, this energy is used to break or form intermolecular bonds, changing the state of the substance.
- Latent Heat Definition
- The energy required to change the state of a substance without changing temperature is called latent heat.
- Latent heat of fusion is used when a solid changes to liquid.
- Latent heat of vaporization is used when a liquid changes to gas.
Example:
- When ice melts at 0°C, it absorbs heat but remains at 0°C until completely melted.
- When water boils at 100°C, it continues to absorb heat without increasing temperature, transforming into steam.
Conclusion:
Temperature remains constant during a change of state because the absorbed or released heat energy is used to alter intermolecular bonding, rather than raising temperature. This ensures a smooth transition between states.
Q. 4. Suggest a method to liquefy atmospheric gases.
Answer:-
Method to Liquefy Atmospheric Gases
Atmospheric gases, such as oxygen, nitrogen, and carbon dioxide, exist in the gaseous state under normal conditions. To convert them into the liquid state, they must be subjected to cooling and compression.
Principle Used: Cooling and Compression
- Compression of Gases
- When gases are compressed, their molecules come closer together, reducing the space between them.
- As pressure increases, the gas moves toward a state where it can change into a liquid.
- Cooling the Compressed Gas
- After compression, the gas is cooled by lowering the temperature.
- When the temperature drops below the gas’s critical temperature, it undergoes liquefaction.
Common Liquefaction Method: Linde’s Process
- Step 1: Atmospheric gas is compressed under high pressure.
- Step 2: It is then cooled by rapid expansion, which lowers its temperature.
- Step 3: This process is repeated multiple times, progressively cooling the gas until it liquefies.
Example:
- Liquid oxygen and liquid nitrogen are produced by cooling and compressing air using this technique.
Conclusion:
Atmospheric gases can be liquefied using a combination of high pressure (compression) and low temperature (cooling). This method is widely used in industrial applications to store gases in their liquid form.
NCERT Textbook for Class 9 Science – Page 10
Q. 1 Why does a desert cooler cool better on a hot dry day?
Answer:-
Why Does a Desert Cooler Work More Efficiently on a Hot Dry Day?
A desert cooler functions based on the principle of evaporative cooling, which is most effective when the air is hot and dry.
1. Role of Evaporation in Cooling
- Evaporation occurs when water absorbs heat from the surroundings and changes into vapor.
- The process lowers the temperature of the air, providing a cooling effect.
2. Effect of Hot and Dry Air
- On a hot dry day, the humidity level is low, allowing faster evaporation.
- As the cooler pumps water over its pads, dry air passing through absorbs more moisture, leading to greater cooling.
3. Contrast with Humid Conditions
- On humid days, air already contains a lot of moisture, reducing the rate of evaporation.
- This makes the cooling effect less effective compared to hot, dry conditions.
Example in Daily Life:
- Sweating cools the body because sweat evaporates faster in dry air, reducing body heat.
- Wet clothes dry quickly in dry weather due to increased evaporation.
Conclusion
A desert cooler works best on hot dry days because lower humidity allows rapid evaporation, leading to efficient cooling.
Q. 2 How does the water kept in an earthen pot (matka) become cool during summer?
Answer:
How Does Water in an Earthen Pot (Matka) Become Cool in Summer?
Water stored in an earthen pot (matka) remains cool during summer due to the process of evaporation and the porous nature of the pot.
1. Porous Structure of the Pot
- The earthen pot is made of clay, which has tiny pores that allow water to slowly seep through.
- As the water reaches the outer surface, it spreads and evaporates into the surrounding air.
2. Cooling Due to Evaporation
- During evaporation, water molecules absorb heat from the remaining water to change into vapor.
- This absorption of heat lowers the temperature of the water inside the pot, making it cooler than the surroundings.
3. Effectiveness in Summer
- In hot and dry weather, evaporation occurs rapidly, enhancing the cooling effect.
- This is why earthen pots are widely used in summers to keep drinking water cool naturally.
Example in Daily Life:
- Sweating cools the body because sweat evaporates and removes heat.
- Desert coolers work best in dry weather due to rapid evaporation.
Conclusion
Water in an earthen pot becomes cool during summer due to evaporative cooling facilitated by the porous structure of the pot. More evaporation leads to better cooling, making it an effective traditional cooling method.
Q. 3 Why does our palm feel cold when we put some acetone or petrol or perfume on it.
Answer:-
Why Does Our Palm Feel Cold When We Apply Acetone, Petrol, or Perfume?
The cooling sensation experienced when acetone, petrol, or perfume is applied to the palm is due to the process of evaporation.
1. Evaporation Causes Cooling
- When a liquid evaporates, its molecules absorb heat from the surroundings to change into vapor.
- This heat is taken from the surface of the palm, leading to a cooling effect.
2. Low Boiling Point of Acetone, Petrol, and Perfume
- These substances have a low boiling point, meaning they evaporate quickly when exposed to air.
- Faster evaporation results in greater heat absorption, making the palm feel cooler.
3. Example of Evaporative Cooling in Daily Life
- Sweating cools the body because sweat absorbs heat from the skin and evaporates.
- Water sprinkled on a hot floor evaporates and cools the surface.
Conclusion
The cooling sensation when acetone, petrol, or perfume is applied is due to evaporation, where the liquid absorbs heat from the palm to change into vapor, resulting in a cooling effect.
Q. 4. Why are we able to slip hot tea or milk faster from a saucer rather than a cup?
Answer:-
Why Can We Sip Hot Tea or Milk Faster from a Saucer than a Cup?
The reason hot tea or milk cools faster in a saucer compared to a cup is due to the concept of surface area and evaporation.
1. Increased Surface Area in a Saucer
- A saucer has a wider surface area compared to a cup.
- More surface exposure allows heat to dissipate faster into the surrounding air.
2. Faster Evaporation
- Due to the larger exposed surface, more molecules escape as vapor.
- The greater rate of evaporation helps in faster cooling, making the liquid easier to sip.
3. Cup vs. Saucer Comparison
- In a cup, the liquid remains deep and compact, reducing the surface area available for heat dissipation.
- In a saucer, the liquid is spread out, allowing better heat transfer to the air.
Example in Daily Life:
- Water spread over a table evaporates faster than water stored in a narrow glass.
- Hot soup cools faster in a wide bowl than in a tall mug.
Conclusion:
Tea or milk cools faster in a saucer due to its larger surface area, which enhances heat dissipation and evaporation, making it easier to sip.
Q. 5. Why type of clothes should be wear in summer?
Answer:-
Recommended Clothing for Summer
During summer, it is important to wear light and breathable clothing to stay comfortable and cool. The choice of clothes should be based on the following factors:
1. Light-Colored Clothes
- Light colors, such as white, pastel shades, or light blue, reflect sunlight and help in keeping the body cool.
- Dark-colored clothes absorb more heat, making them uncomfortable in hot weather.
2. Cotton and Loose-Fitting Clothes
- Cotton fabric absorbs sweat and allows it to evaporate, keeping the body dry.
- Loose-fitting clothes allow air circulation, preventing excessive sweating and discomfort.
3. Breathable Fabrics
- Natural fibers like cotton and linen permit airflow, making them suitable for summer.
- Synthetic fabrics trap heat, leading to discomfort.
4. Sun Protection Accessories
- Wearing a hat or carrying an umbrella protects the skin from direct sunlight.
- Sunglasses safeguard the eyes from harmful UV rays.
Conclusion
For summer, it is best to wear light-colored, cotton, and loose-fitting clothes to stay cool and comfortable. Avoid dark and synthetic fabrics, and consider sun protection accessories to minimize the effects of heat.
(Chapter – 1) [Matter in our Surroundings] Back Excercises
Q. 1. Convery the following temperatures to the Celsius scale.
(a) 293 K
(b) 470 K
Answer:-
To convert temperatures from Kelvin (K) to Celsius (°C), use the formula:
Celsius = [ Temperature in Kelvin – 273 ]
Now, applying this formula:
(a) 293 K to °C
[ 293 – 273 = 20o C ]
(b) 470 K to °C
[ 470 – 273 = 197o C ]
Thus, the converted temperatures are:
- 293 K = 20°C
- 470 K = 197°C
Q. 2. Convert the following temperatures to the kelvin scale.
(a) 25o C
(b) 373o C
Answer:-
To convert temperatures from Celsius (°C) to Kelvin (K), use the formula:
[ Temperature in Kelvin = [ Temperature in Celsius + 273 ]
Now, applying this formula:
(a) 25°C to K
[ 25 + 273 = 298 K ]
(b) 373°C to K
[ 373 + 273 = 646 K ]
Thus, the converted temperatures are:
- 25°C = 298 K
- 373°C = 646 K
Q. 3. Give reason for the following observations:
(a) Naphthalene balls disappear with time without leaving any solid.
Answer:-
Reason for Observation: Naphthalene Balls Disappear Over Time
Naphthalene balls disappear with time without leaving any solid because they undergo sublimation.
Explanation:
- Sublimation is the process in which a solid directly changes into a gas without passing through the liquid state.
- Naphthalene is a volatile substance, meaning its molecules can escape easily into the air.
- When exposed to air, naphthalene balls gradually sublimate, turning into vapors without forming a liquid residue.
- As a result, they shrink in size over time and eventually disappear completely.
(b) We can get the smell of perfume sitting several metres away.
Answer:-
Reason for Observation: Smelling Perfume from a Distance
We can get the smell of perfume sitting several meters away because perfume vapors diffuse in air.
Explanation:
- Diffusion is the process in which particles move from a region of higher concentration to a region of lower concentration.
- Perfume contains volatile molecules that evaporate quickly and mix with air.
- These molecules spread out and move in all directions due to diffusion.
- As a result, the smell of perfume reaches people sitting far away, even without direct contact.
Q. 4. Arrange the following substances in increasing order of forces of attraction between the particles – Water, Sugar, Oxygen.
Answer:-
Arranging Substances Based on Forces of Attraction
The force of attraction between particles varies depending on the state of matter—solids have the strongest intermolecular forces, followed by liquids, and gases have the weakest.
Given substances:
- Oxygen (O₂) → Gas (weakest force of attraction)
- Water (H₂O) → Liquid (moderate force of attraction)
- Sugar (C₁₂H₂₂O₁₁) → Solid (strongest force of attraction)
Thus, the increasing order of forces of attraction is:
Oxygen < Water < Sugar
Q. 5. What is the physical state of water at —
(a) 25o C
(b) 0o C
(c) 100o C
Answer:-
Physical State of Water at Different Temperatures
The physical state of water depends on its temperature:
(a) At 25°C → Liquid State
- Water remains in its liquid form at room temperature (25°C).
(b) At 0°C → Solid and Liquid Coexist
- 0°C is the freezing/melting point of water.
- At this temperature, water exists as both ice (solid) and liquid in equilibrium.
(c) At 100°C → Liquid and Gas Coexist
- 100°C is the boiling point of water.
- At this temperature, liquid water starts converting into steam (gas) while some liquid still remains.
Final Answer:
- 25°C → Liquid
- 0°C → Solid & Liquid coexist
- 100°C → Liquid & Gas coexist
Q. 6. Give two reasons to justify —
(a) Water at room temperature is a liquid.
Answer:-
Reasons Why Water at Room Temperature is a Liquid
Water remains in the liquid state at room temperature (approximately 25°C) due to the following reasons:
1. Moderate Intermolecular Forces
- Water molecules experience moderate forces of attraction (hydrogen bonding).
- These forces are strong enough to keep the molecules close together, but not strong enough to fix them in a solid structure.
- This allows water to flow and take the shape of its container, characteristic of a liquid.
2. Melting and Boiling Points
- Water has a freezing point of 0°C and a boiling point of 100°C.
- Since room temperature (25°C) lies between these two points, water does not freeze into a solid or evaporate into a gas.
- Instead, it remains in the liquid state at this temperature.
Final Answer:
Water is a liquid at room temperature because:
- It has moderate intermolecular forces allowing molecular movement.
- Its freezing and boiling points ensure it stays liquid at 25°C.
Make sure to include the reasoning clearly in your answer to secure full marks in your Class 9 board exams! Let me know if you need any further clarification. 😊
(b) an iron almirah is a solid at room temperature.
Answer:-
Reasons Why an Iron Almirah is a Solid at Room Temperature
An iron almirah remains solid at room temperature (~25°C) due to the following reasons:
1. Strong Intermolecular Forces
- Iron is a metal with strong metallic bonds between its atoms.
- These forces hold the atoms tightly in a rigid structure, preventing free movement like in liquids or gases.
- As a result, the iron almirah maintains a definite shape and volume.
2. High Melting Point
- Iron has a very high melting point (around 1538°C).
- Since room temperature (25°C) is much lower than this melting point, iron does not melt and remains solid.
- It does not flow like liquids or expand freely like gases.
Final Answer:
An iron almirah is solid at room temperature because:
- It has strong intermolecular forces that keep it rigid.
- Its high melting point prevents it from melting at 25°C.
Q. 7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer:-
Why Ice at 273 K is More Effective in Cooling than Water at the Same Temperature
Ice at 273 K (0°C) is more effective in cooling than water at the same temperature due to latent heat absorption.
1. Latent Heat of Fusion
- When ice melts into water at 273 K, it absorbs latent heat from its surroundings.
- This extra energy absorption (latent heat of fusion = 334 J/g) makes ice cooler and more effective in lowering the temperature of nearby objects.
2. Water at 273 K Lacks Latent Heat Absorption
- Water at 273 K is already in the liquid state and does not need extra energy to change phase.
- Since it does not absorb latent heat, it does not provide the same cooling effect as ice.
Final Answer:
Ice at 273 K is more effective in cooling than water at the same temperature because:
- It absorbs latent heat (334 J/g) while melting, further reducing surrounding temperature.
- Water at 273 K does not require latent heat absorption, so it does not cool as much.
Q. 8. What produces more severe burns, boiling water or steam?
Answer:-
Steam vs. Boiling Water: Which Causes More Severe Burns?
Steam produces more severe burns than boiling water at the same temperature (100°C). This happens due to latent heat release and deeper skin penetration.
1. Latent Heat of Vaporization
- Steam has extra stored energy because it has already absorbed latent heat of vaporization to convert from liquid water to gas.
- When steam condenses back into water on the skin, it releases this extra heat, causing more damage than boiling water.
2. Deeper Penetration into Skin
- Steam particles spread quickly, allowing them to penetrate deeper layers of skin compared to boiling water.
- This leads to more tissue damage, making steam burns more painful and severe.
Final Answer:
Steam causes more severe burns than boiling water because:
- It releases latent heat when it condenses.
- It penetrates deeper into the skin, causing greater damage.
Q. 9. Name A, B, C, D, E and F in the following diagram showing change in its state.
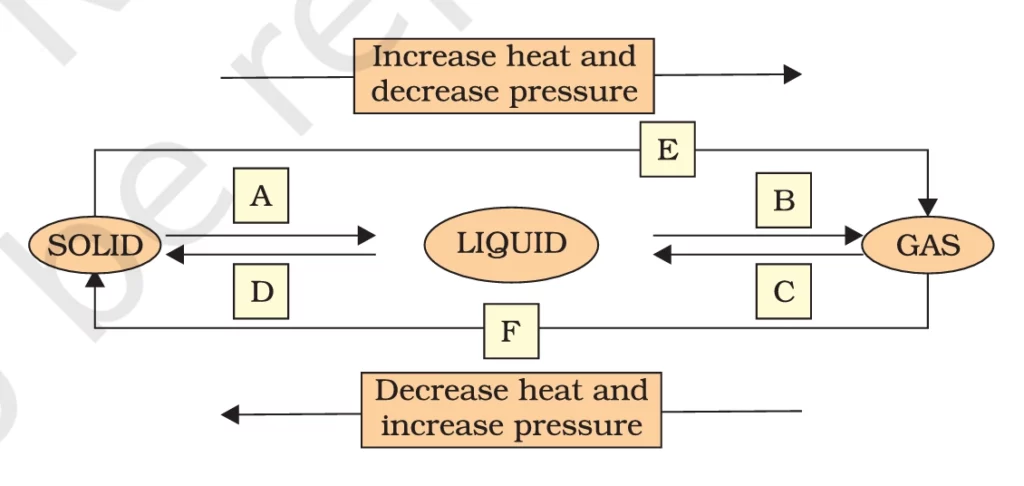
Answer:-
Thanks for pointing that out! Let’s correct it:
In the diagram showing the change in states of matter, the correct labels should be:
- A: Melting → Solid to Liquid
- B: Evaporation → Liquid to Gas
- C: Condensation → Gas to Liquid
- D: Freezing → Liquid to Solid
- E: Sublimation → Solid to Gas
- F: Deposition → Gas to Solid